当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning Cu/Cu2O Interfaces for the Reduction of Carbon Dioxide to Methanol in Aqueous Solutions
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-31 , DOI: 10.1002/anie.201805256 Xiaoxia Chang 1 , Tuo Wang 1 , Zhi‐Jian Zhao 1 , Piaoping Yang 1 , Jeffrey Greeley 2 , Rentao Mu 1 , Gong Zhang 1 , Zhongmiao Gong 3 , Zhibin Luo 1 , Jun Chen 4 , Yi Cui 3 , Geoffrey A. Ozin 5 , Jinlong Gong 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-31 , DOI: 10.1002/anie.201805256 Xiaoxia Chang 1 , Tuo Wang 1 , Zhi‐Jian Zhao 1 , Piaoping Yang 1 , Jeffrey Greeley 2 , Rentao Mu 1 , Gong Zhang 1 , Zhongmiao Gong 3 , Zhibin Luo 1 , Jun Chen 4 , Yi Cui 3 , Geoffrey A. Ozin 5 , Jinlong Gong 1
Affiliation
|
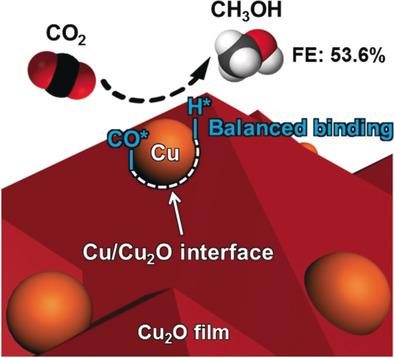
Artificial photosynthesis can be used to store solar energy and reduce CO2 into fuels to potentially alleviate global warming and the energy crisis. Compared to the generation of gaseous products, it remains a great challenge to tune the product distribution of artificial photosynthesis to liquid fuels, such as CH3OH, which are suitable for storage and transport. Herein, we describe the introduction of metallic Cu nanoparticles (NPs) on Cu2O films to change the product distribution from gaseous products on bare Cu2O to predominantly CH3OH by CO2 reduction in aqueous solutions. The specifically designed Cu/Cu2O interfaces balance the binding strengths of H* and CO* intermediates, which play critical roles in CH3OH production. With a TiO2 model photoanode to construct a photoelectrochemical cell, a Cu/Cu2O dark cathode exhibited a Faradaic efficiency of up to 53.6 % for CH3OH production. This work demonstrates the feasibility and mechanism of interface engineering to enhance the CH3OH production from CO2 reduction in aqueous electrolytes.
中文翻译:

调整Cu / Cu2O界面以将二氧化碳还原为水溶液中的甲醇
人工光合作用可用于存储太阳能并减少CO 2转化为燃料,从而有可能缓解全球变暖和能源危机。与气态产物的产生相比,调整人造光合作用到适合于存储和运输的液体燃料(例如CH 3 OH)的产物分布仍然是一个巨大的挑战。在这里,我们描述了在Cu 2 O膜上引入金属Cu纳米颗粒(NPs),以通过水溶液中的CO 2还原将产物分布从裸露的Cu 2 O上的气态产物变为主要为CH 3 OH 。专门设计的Cu / Cu 2O界面平衡了H *和CO *中间体的结合强度,这在CH 3 OH的生产中起着至关重要的作用。用TiO 2模型光电阳极构建光电化学电池,Cu / Cu 2 O暗阴极对CH 3 OH的生产具有高达53.6%的法拉第效率。这项工作证明了界面工程提高水性电解质中CO 2还原产生CH 3 OH产量的可行性和机理。
更新日期:2018-10-31
中文翻译:

调整Cu / Cu2O界面以将二氧化碳还原为水溶液中的甲醇
人工光合作用可用于存储太阳能并减少CO 2转化为燃料,从而有可能缓解全球变暖和能源危机。与气态产物的产生相比,调整人造光合作用到适合于存储和运输的液体燃料(例如CH 3 OH)的产物分布仍然是一个巨大的挑战。在这里,我们描述了在Cu 2 O膜上引入金属Cu纳米颗粒(NPs),以通过水溶液中的CO 2还原将产物分布从裸露的Cu 2 O上的气态产物变为主要为CH 3 OH 。专门设计的Cu / Cu 2O界面平衡了H *和CO *中间体的结合强度,这在CH 3 OH的生产中起着至关重要的作用。用TiO 2模型光电阳极构建光电化学电池,Cu / Cu 2 O暗阴极对CH 3 OH的生产具有高达53.6%的法拉第效率。这项工作证明了界面工程提高水性电解质中CO 2还原产生CH 3 OH产量的可行性和机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号