当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO Chemical Capture on Lithium Cuprate, Through a Consecutive CO Oxidation and Chemisorption Bifunctional Process
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-17 00:00:00 , DOI: 10.1021/acs.jpcc.5b11147 Hugo Lara-Garcia 1 , Brenda Alcántar-Vázquez 1 , Yuhua Duan 2 , Heriberto Pfeiffer 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-17 00:00:00 , DOI: 10.1021/acs.jpcc.5b11147 Hugo Lara-Garcia 1 , Brenda Alcántar-Vázquez 1 , Yuhua Duan 2 , Heriberto Pfeiffer 1
Affiliation
|
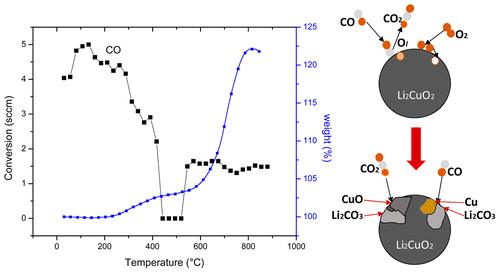
Lithium cuprate was studied as a possible catalytic and captor material for CO oxidation and the subsequent CO2 chemisorption process, a bifunctional material. The CO oxidation reaction was analyzed in a high temperature reactor coupled to an FTIR spectrometer and a gas chromatograph. The CO2 production and consequent chemisorption processes were analyzed by thermogravimetric analysis. All of these experiments were performed in both the presence and absence of oxygen. Experimental results were then compared with theoretical thermodynamic data. The results clearly showed that Li2CuO2 can be used as a bifunctional material; it is able to catalyze the CO oxidation, producing CO2, and, subsequently, it can chemically capture the CO2. Both processes occur in the presence or absence of oxygen and follow the Mars–van Krevelen reaction mechanism.
中文翻译:

通过连续的CO氧化和化学吸附双功能过程在铜酸锂上捕获CO化学物质
研究了铜酸锂作为一种可能的催化和捕获剂,用于CO氧化和随后的CO 2化学吸附过程,这是一种双功能材料。在与FTIR光谱仪和气相色谱仪偶联的高温反应器中分析CO氧化反应。通过热重分析来分析CO 2的产生和随后的化学吸附过程。所有这些实验均在有氧和无氧条件下进行。然后将实验结果与理论热力学数据进行比较。结果清楚地表明,Li 2 CuO 2可以用作双功能材料。它能够催化CO氧化,产生CO 2并随后可以化学捕获CO 2。这两个过程都在有氧或无氧条件下发生,并遵循Mars-van Krevelen反应机理。
更新日期:2016-02-17
中文翻译:

通过连续的CO氧化和化学吸附双功能过程在铜酸锂上捕获CO化学物质
研究了铜酸锂作为一种可能的催化和捕获剂,用于CO氧化和随后的CO 2化学吸附过程,这是一种双功能材料。在与FTIR光谱仪和气相色谱仪偶联的高温反应器中分析CO氧化反应。通过热重分析来分析CO 2的产生和随后的化学吸附过程。所有这些实验均在有氧和无氧条件下进行。然后将实验结果与理论热力学数据进行比较。结果清楚地表明,Li 2 CuO 2可以用作双功能材料。它能够催化CO氧化,产生CO 2并随后可以化学捕获CO 2。这两个过程都在有氧或无氧条件下发生,并遵循Mars-van Krevelen反应机理。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号