当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic and Electronic Structure Study of ETHE1: Elucidating the Factors Influencing Sulfur Oxidation and Oxygenation in Mononuclear Non-heme Iron Enzymes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-16 , DOI: 10.1021/jacs.8b09022 Serra Goudarzi 1 , Jeffrey T. Babicz 1 , Omer Kabil 2 , Ruma Banerjee 2 , Edward I. Solomon 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-16 , DOI: 10.1021/jacs.8b09022 Serra Goudarzi 1 , Jeffrey T. Babicz 1 , Omer Kabil 2 , Ruma Banerjee 2 , Edward I. Solomon 1, 3
Affiliation
|
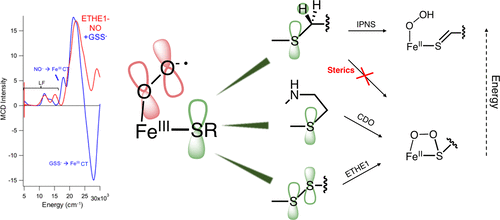
ETHE1 is a member of a growing subclass of nonheme Fe enzymes that catalyzes transformations of sulfur-containing substrates without a cofactor. ETHE1 dioxygenates glutathione persulfide (GSSH) to glutathione (GSH) and sulfite in a reaction which is similar to that of cysteine dioxygenase (CDO), but with monodentate (vs bidentate) substrate coordination and a 2-His/1-Asp (vs 3-His) ligand set. In this study, we demonstrate that GSS- binds directly to the iron active site, causing coordination unsaturation to prime the site for O2 activation. Nitrosyl complexes without and with GSSH were generated and spectroscopically characterized as unreactive analogues for the invoked ferric superoxide intermediate. New spectral features from persulfide binding to the FeIII include the appearance of a low-energy FeIII ligand field transition, an energy shift of a NO- to FeIII CT transition, and two new GSS- to FeIII CT transitions. Time-dependent density functional theory calculations were used to simulate the experimental spectra to determine the persulfide orientation. Correlation of these spectral features with those of monodentate cysteine binding in isopenicillin N synthase (IPNS) shows that the persulfide is a poorer donor but still results in an equivalent frontier molecular orbital for reactivity. The ETHE1 persulfide dioxygenation reaction coordinate was calculated, and while the initial steps are similar to the reaction coordinate of CDO, an additional hydrolysis step is required in ETHE1 to break the S-S bond. Unlike ETHE1 and CDO, which both oxygenate sulfur, IPNS oxidizes sulfur through an initial H atom abstraction. Thus, factors that determine oxygenase vs oxidase reactivity were evaluated. In general, sulfur oxygenation is thermodynamically favored and has a lower barrier for reactivity. However, in IPNS, second-sphere residues in the active site pocket constrain the substrate, raising the barrier for sulfur oxygenation relative to oxidation via H atom abstraction.
中文翻译:

ETHE1的光谱和电子结构研究:阐明影响单核非血红素铁酶中硫氧化和氧化的因素
ETHE1 是一个不断增长的非血红素 Fe 酶亚类的成员,该酶催化含硫底物的转化,无需辅因子。ETHE1 在与半胱氨酸双加氧酶 (CDO) 相似的反应中将谷胱甘肽过硫化物 (GSSH) 双氧合为谷胱甘肽 (GSH) 和亚硫酸盐,但具有单齿 (vs 双齿) 底物配位和 2-His/1-Asp (vs 3 -His) 配体组。在这项研究中,我们证明 GSS- 直接与铁活性位点结合,导致配位不饱和度为 O2 激活位点做好准备。产生和没有 GSSH 的亚硝基配合物,并在光谱上表征为调用的超氧化铁中间体的非反应性类似物。过硫化物与 FeIII 结合的新光谱特征包括低能 FeIII 配体场跃迁的出现,NO- 到 FeIII CT 跃迁的能量转移,以及两个新的 GSS 到 FeIII CT 跃迁。时间相关的密度泛函理论计算用于模拟实验光谱以确定过硫化物的取向。这些光谱特征与异青霉素 N 合酶 (IPNS) 中单齿半胱氨酸结合的光谱特征的相关性表明,过硫化物是较差的供体,但仍导致反应性的等效前沿分子轨道。计算了 ETHE1 过硫化物双加氧反应坐标,虽然初始步骤类似于 CDO 的反应坐标,但在 ETHE1 中需要额外的水解步骤来破坏 SS 键。与都氧化硫的 ETHE1 和 CDO 不同,IPNS 通过初始 H 原子提取氧化硫。因此,评估了决定加氧酶与氧化酶反应性的因素。通常,硫氧化在热力学上是有利的,并且对反应性具有较低的屏障。然而,在 IPNS 中,活性位点口袋中的第二球残基限制了底物,相对于通过 H 原子提取进行的氧化,提高了硫氧化的障碍。
更新日期:2018-10-16
中文翻译:

ETHE1的光谱和电子结构研究:阐明影响单核非血红素铁酶中硫氧化和氧化的因素
ETHE1 是一个不断增长的非血红素 Fe 酶亚类的成员,该酶催化含硫底物的转化,无需辅因子。ETHE1 在与半胱氨酸双加氧酶 (CDO) 相似的反应中将谷胱甘肽过硫化物 (GSSH) 双氧合为谷胱甘肽 (GSH) 和亚硫酸盐,但具有单齿 (vs 双齿) 底物配位和 2-His/1-Asp (vs 3 -His) 配体组。在这项研究中,我们证明 GSS- 直接与铁活性位点结合,导致配位不饱和度为 O2 激活位点做好准备。产生和没有 GSSH 的亚硝基配合物,并在光谱上表征为调用的超氧化铁中间体的非反应性类似物。过硫化物与 FeIII 结合的新光谱特征包括低能 FeIII 配体场跃迁的出现,NO- 到 FeIII CT 跃迁的能量转移,以及两个新的 GSS 到 FeIII CT 跃迁。时间相关的密度泛函理论计算用于模拟实验光谱以确定过硫化物的取向。这些光谱特征与异青霉素 N 合酶 (IPNS) 中单齿半胱氨酸结合的光谱特征的相关性表明,过硫化物是较差的供体,但仍导致反应性的等效前沿分子轨道。计算了 ETHE1 过硫化物双加氧反应坐标,虽然初始步骤类似于 CDO 的反应坐标,但在 ETHE1 中需要额外的水解步骤来破坏 SS 键。与都氧化硫的 ETHE1 和 CDO 不同,IPNS 通过初始 H 原子提取氧化硫。因此,评估了决定加氧酶与氧化酶反应性的因素。通常,硫氧化在热力学上是有利的,并且对反应性具有较低的屏障。然而,在 IPNS 中,活性位点口袋中的第二球残基限制了底物,相对于通过 H 原子提取进行的氧化,提高了硫氧化的障碍。































 京公网安备 11010802027423号
京公网安备 11010802027423号