当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Organic Matter on Iron(II)-Catalyzed Mineral Transformations in Ferrihydrite–Organic Matter Coprecipitates
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-10-16 , DOI: 10.1021/acs.est.8b03206 Laurel K. ThomasArrigo 1 , James M. Byrne 2 , Andreas Kappler 2 , Ruben Kretzschmar 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-10-16 , DOI: 10.1021/acs.est.8b03206 Laurel K. ThomasArrigo 1 , James M. Byrne 2 , Andreas Kappler 2 , Ruben Kretzschmar 1
Affiliation
|
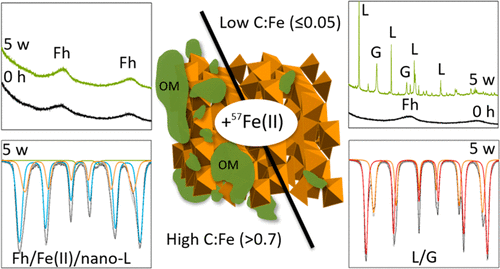
Poorly crystalline Fe(III) (oxyhydr)oxides like ferrihydrite are abundant in soils and sediments and are often associated with organic matter (OM) in the form of mineral-organic aggregates. Under anoxic conditions, interactions between aqueous Fe(II) and ferrihydrite lead to the formation of crystalline secondary minerals, like lepidocrocite, goethite, or magnetite. However, the extent to which Fe(II)-catalyzed mineral transformations are influenced by ferrihydrite-associated OM is not well understood. We therefore reacted ferrihydrite-PGA coprecipitates (PGA = polygalacturonic acid, C:Fe molar ratios = 0–2.5) and natural Fe-rich organic flocs (C:Fe molar ratio = 2.2) with 0.5–5.0 mM isotopically labeled 57Fe(II) at pH 7 for 5 weeks. Relying on the combination of stable Fe isotope tracers, a novel application of the PONKCS method to Rietveld fitting of X-ray diffraction (XRD) patterns, and 57Fe Mössbauer spectroscopy, we sought to follow the temporal evolution in Fe mineralogy and elucidate the fate of adsorbed 57Fe(II). At low C:Fe molar ratios (0–0.05), rapid oxidation of surface-adsorbed 57Fe(II) resulted in 57Fe-enriched crystalline minerals and nearly complete mineral transformation within days. With increasing OM content, the atom exchange between the added aqueous 57Fe(II) and Fe in the organic-rich solids still occurred; however, XRD analysis showed that crystalline mineral precipitation was strongly inhibited. For high OM-content materials (C:Fe ≥ 1.2), Mössbauer spectroscopy revealed up to 39% lepidocrocite in the final Fe(II)-reacted samples. Because lepidocrocite was not detectable by XRD, we suggest that the Mössbauer-detected lepidocrocite consisted of nanosized clusters with lepidocrocite-like local structure, similar to the lepidocrocite found in natural flocs. Collectively, our results demonstrate that the C content of ferrihydrite–OM coprecipitates strongly impacts the degree and pathways of Fe mineral transformations and iron atom exchange during reactions with aqueous Fe(II).
中文翻译:

有机物对铁(II)催化的水铁矿-有机物共沉淀物中矿物转化的影响
结晶度较弱的Fe(III)(羟基)氧化物如亚铁酸盐在土壤和沉积物中含量很高,并且经常以矿物-有机聚集体的形式与有机物(OM)结合。在缺氧条件下,Fe(II)水溶液与亚铁酸盐之间的相互作用导致形成结晶性次生矿物,如纤铁矿,针铁矿或磁铁矿。但是,人们还不太了解Fe(II)催化的矿物转化受亚铁水合物相关OM的影响。因此,我们使水铁矿-PGA共沉淀物(PGA =聚半乳糖醛酸,C:Fe摩尔比= 0–2.5)和天然富铁有机絮凝物(C:Fe摩尔比= 2.2)与0.5–5.0 mM同位素标记57Fe(II)在pH 7下持续5周。依靠稳定的Fe同位素示踪剂的组合,PONKCS方法在X射线衍射(XRD)图的Rietveld拟合和57 FeMössbauer光谱学上的新应用,我们试图追踪Fe矿物学的时间演变并阐明其命运吸附57 Fe(II)。在低的C:Fe摩尔比(0-0.05)下,表面吸附的57 Fe(II)的快速氧化导致57 Fe富集的结晶矿物,并在几天内几乎完成了矿物转化。随着OM含量的增加,添加的水溶液57之间的原子交换富含有机物的固体中仍存在Fe(II)和Fe;然而,XRD分析表明,晶体矿物沉淀被强烈抑制。对于高OM含量的材料(C:Fe≥1.2),穆斯堡尔光谱法显示最终Fe(II)反应的样品中高达39%的纤铁矿。由于XRD无法检测到纤铁矿,因此我们建议由Mössbauer检测到的纤铁矿由具有类似于纤铁矿的局部结构的纳米簇组成,类似于在天然絮凝物中发现的纤铁矿。总的来说,我们的结果表明,水铁矿-OM中的C含量会在与Fe(II)水溶液反应期间强烈影响Fe矿物转化和铁原子交换的程度和途径。
更新日期:2018-10-16
中文翻译:

有机物对铁(II)催化的水铁矿-有机物共沉淀物中矿物转化的影响
结晶度较弱的Fe(III)(羟基)氧化物如亚铁酸盐在土壤和沉积物中含量很高,并且经常以矿物-有机聚集体的形式与有机物(OM)结合。在缺氧条件下,Fe(II)水溶液与亚铁酸盐之间的相互作用导致形成结晶性次生矿物,如纤铁矿,针铁矿或磁铁矿。但是,人们还不太了解Fe(II)催化的矿物转化受亚铁水合物相关OM的影响。因此,我们使水铁矿-PGA共沉淀物(PGA =聚半乳糖醛酸,C:Fe摩尔比= 0–2.5)和天然富铁有机絮凝物(C:Fe摩尔比= 2.2)与0.5–5.0 mM同位素标记57Fe(II)在pH 7下持续5周。依靠稳定的Fe同位素示踪剂的组合,PONKCS方法在X射线衍射(XRD)图的Rietveld拟合和57 FeMössbauer光谱学上的新应用,我们试图追踪Fe矿物学的时间演变并阐明其命运吸附57 Fe(II)。在低的C:Fe摩尔比(0-0.05)下,表面吸附的57 Fe(II)的快速氧化导致57 Fe富集的结晶矿物,并在几天内几乎完成了矿物转化。随着OM含量的增加,添加的水溶液57之间的原子交换富含有机物的固体中仍存在Fe(II)和Fe;然而,XRD分析表明,晶体矿物沉淀被强烈抑制。对于高OM含量的材料(C:Fe≥1.2),穆斯堡尔光谱法显示最终Fe(II)反应的样品中高达39%的纤铁矿。由于XRD无法检测到纤铁矿,因此我们建议由Mössbauer检测到的纤铁矿由具有类似于纤铁矿的局部结构的纳米簇组成,类似于在天然絮凝物中发现的纤铁矿。总的来说,我们的结果表明,水铁矿-OM中的C含量会在与Fe(II)水溶液反应期间强烈影响Fe矿物转化和铁原子交换的程度和途径。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号