当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into Transannular Cyclization Reactions To Synthesize Azabicyclo[X.Y.Z]alkanone Amino Acid Derivatives from 8-, 9-, and 10-Membered Macrocyclic Dipeptide Lactams
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2015-04-29 00:00:00 , DOI: 10.1021/acs.joc.5b00237 N. D. Prasad Atmuri 1 , William D. Lubell 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2015-04-29 00:00:00 , DOI: 10.1021/acs.joc.5b00237 N. D. Prasad Atmuri 1 , William D. Lubell 1
Affiliation
|
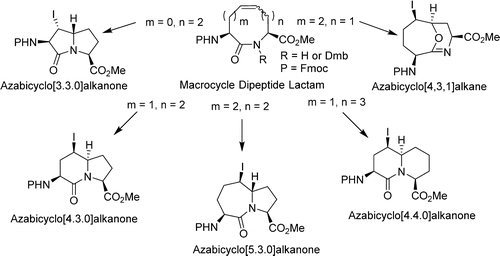
An efficient method for synthesizing different functionalized azabicyclo[X.Y.0]alkanone amino acid derivatives has been developed employing electrophilic transannular cyclizations of 8-, 9-, and 10-membered unsaturated macrocycles to form 5,5-, 6,5-, 7,5-, and 6,6-fused bicylic amino acids, respectively. Macrocycles were obtained by a sequence featuring peptide coupling of vinyl-, allyl-, homoallyl-, and homohomoallylglycine building blocks followed by ring-closing metathesis. X-ray crystallographic analyses of the 8-, 9-, and 10-membered macrocyclic lactam starting materials as well as certain bicyclic amino acid products provided insight into their conformational preferences as well as the mechanism for the diastereoselective formation of specific azabicycloalkanone amino acids by way of transannular iodolactamization reactions.
中文翻译:

跨环环化反应合成Azabicyclo [ X的见解。ÿ。8元,9元和10元大环二肽内酰胺的Z ]烷酮氨基酸衍生物
一种合成不同功能化氮杂双环[ X的有效方法。ÿ.0]烷酮氨基酸衍生物是利用8、9和10元不饱和大环的亲电跨环环化反应形成5、5、6、5、7、5和6,6稠合的双环氨基酸。通过以乙烯基-,烯丙基-,高烯丙基-和高同烯丙基甘氨酸结构单元的肽偶联为特征,然后闭环易位的序列获得大环。对8元,9元和10元大环内酰胺起始原料以及某些双环氨基酸产物的X射线晶体学分析提供了对它们的构象偏爱以及非对映选择性形成特定氮杂双环烷酮氨基酸的机理的见解。环戊内酰胺化反应的方式。
更新日期:2015-04-29
中文翻译:

跨环环化反应合成Azabicyclo [ X的见解。ÿ。8元,9元和10元大环二肽内酰胺的Z ]烷酮氨基酸衍生物
一种合成不同功能化氮杂双环[ X的有效方法。ÿ.0]烷酮氨基酸衍生物是利用8、9和10元不饱和大环的亲电跨环环化反应形成5、5、6、5、7、5和6,6稠合的双环氨基酸。通过以乙烯基-,烯丙基-,高烯丙基-和高同烯丙基甘氨酸结构单元的肽偶联为特征,然后闭环易位的序列获得大环。对8元,9元和10元大环内酰胺起始原料以及某些双环氨基酸产物的X射线晶体学分析提供了对它们的构象偏爱以及非对映选择性形成特定氮杂双环烷酮氨基酸的机理的见解。环戊内酰胺化反应的方式。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号