当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Hydrolyses of Trifluoroacetyl Fluoride by Water
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-10-13 , DOI: 10.1016/j.cplett.2018.10.038 Xinli Song
中文翻译:

水催化三氟乙酰氟的水解
更新日期:2018-10-14
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-10-13 , DOI: 10.1016/j.cplett.2018.10.038 Xinli Song
|
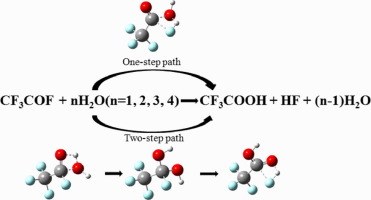
The gas-phase hydrolyses of CF3COF catalyzed by water have been theoretically investigated at the CBS-QB3 level, in which up to four water molecules are considered explicitly and the effect of water bulk solvent is also considered within the polarizable continuum model (PCM). Both stepwise and concerted pathways are identified for hydrolyses of CF3COF, which are competitive with the increasing number of the water molecules. The activation Gibbs free energies are decreased from about 40 kcal mol-1 to 20 kcal mol-1 due to the catalytic effect of water molecules.
中文翻译:

水催化三氟乙酰氟的水解
在CBS-QB3级别上已对水催化CF 3 COF的气相水解进行了理论研究,其中明确考虑了多达四个水分子,并且在可极化连续体模型(PCM)中也考虑了水溶剂的影响)。确定了CF 3 COF水解的逐步途径和协同途径,它们与水分子数量的增加竞争。由于水分子的催化作用,活化吉布斯自由能从约40 kcal mol -1降低到20 kcal mol -1。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号