当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-10-12 , DOI: 10.1016/j.ejmech.2018.10.023 Igor A Schepetkin 1 , Andrei I Khlebnikov 2 , Andrei S Potapov 3 , Anastasia R Kovrizhina 3 , Vladislava V Matveevskaya 4 , Maxim L Belyanin 3 , Dmitriy N Atochin 5 , Svitlana O Zanoza 6 , Nadiya M Gaidarzhy 6 , Sergiy A Lyakhov 6 , Liliya N Kirpotina 1 , Mark T Quinn 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-10-12 , DOI: 10.1016/j.ejmech.2018.10.023 Igor A Schepetkin 1 , Andrei I Khlebnikov 2 , Andrei S Potapov 3 , Anastasia R Kovrizhina 3 , Vladislava V Matveevskaya 4 , Maxim L Belyanin 3 , Dmitriy N Atochin 5 , Svitlana O Zanoza 6 , Nadiya M Gaidarzhy 6 , Sergiy A Lyakhov 6 , Liliya N Kirpotina 1 , Mark T Quinn 1
Affiliation
|
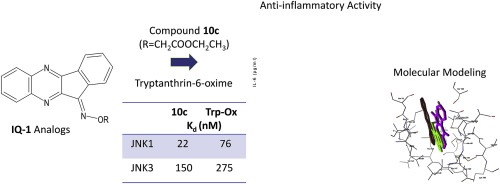
c-Jun N-terminal kinases (JNKs) play a central role in many physiologic and pathologic processes. We synthesized novel 11H-indeno[1,2-b]quinoxalin-11-one oxime analogs and tryptanthrin-6-oxime (indolo[2,1-b]quinazoline-6,12-dion-6-oxime) and evaluated their effects on JNK activity. Several compounds exhibited sub-micromolar JNK binding affinity and were selective for JNK1/JNK3 versus JNK2. The most potent compounds were 10c (11H-indeno[1,2-b]quinoxalin-11-one O-(O-ethylcarboxymethyl) oxime) and tryptanthrin-6-oxime, which had dissociation constants (Kd) for JNK1 and JNK3 of 22 and 76 nM and 150 and 275 nM, respectively. Molecular modeling suggested a mode of binding interaction at the JNK catalytic site and that the selected oxime derivatives were potentially competitive JNK inhibitors. JNK binding activity of the compounds correlated with their ability to inhibit lipopolysaccharide (LPS)-induced nuclear factor-κB/activating protein 1 (NF-κB/AP-1) activation in human monocytic THP-1Blue cells and interleukin-6 (IL-6) production by human MonoMac-6 cells. Thus, oximes with indenoquinoxaline and tryptanthrin nuclei can serve as specific small-molecule modulators for mechanistic studies of JNK, as well as potential leads for the development of anti-inflammatory drugs.
中文翻译:

合成,生物学评估和分子模拟的11 H-茚并[1,2-b]喹喔啉-11-1衍生物和类胰蛋白酶-6-肟作为c-Jun N端激酶抑制剂。
c-Jun N末端激酶(JNK)在许多生理和病理过程中起着核心作用。我们合成了新型11H-茚并[1,2-b]喹喔啉-11-一肟类似物和色胺素6-肟(吲哚[2,1-b]喹唑啉-6,12-dion-6-肟),并对其进行了评估。对JNK活性的影响。几种化合物表现出亚微摩尔的JNK结合亲和力,对JNK1 / JNK3和JNK2具有选择性。最有效的化合物是10c(11H-茚并[1,2-b]喹喔啉-11-一个O-(O-乙基羧甲基)肟)和色胺酮-6-肟,它们的JNK1和JNK3的解离常数(Kd)为分别为22和76 nM,以及150和275 nM。分子建模表明在JNK催化位点的结合相互作用的模式,并且所选的肟衍生物是潜在的竞争性JNK抑制剂。化合物的JNK结合活性与其抑制脂多糖(LPS)诱导的人单核THP-1Blue细胞和白介素6(IL-β)中核因子-κB/活化蛋白1(NF-κB/ AP-1)活化的能力有关。 6)由人的MonoMac-6细胞产生。因此,具有茚并喹喔啉和色胺酮核的肟可以作为JNK机理研究的特定小分子调节剂,并可能成为开发消炎药的潜在先导。
更新日期:2018-10-12
中文翻译:

合成,生物学评估和分子模拟的11 H-茚并[1,2-b]喹喔啉-11-1衍生物和类胰蛋白酶-6-肟作为c-Jun N端激酶抑制剂。
c-Jun N末端激酶(JNK)在许多生理和病理过程中起着核心作用。我们合成了新型11H-茚并[1,2-b]喹喔啉-11-一肟类似物和色胺素6-肟(吲哚[2,1-b]喹唑啉-6,12-dion-6-肟),并对其进行了评估。对JNK活性的影响。几种化合物表现出亚微摩尔的JNK结合亲和力,对JNK1 / JNK3和JNK2具有选择性。最有效的化合物是10c(11H-茚并[1,2-b]喹喔啉-11-一个O-(O-乙基羧甲基)肟)和色胺酮-6-肟,它们的JNK1和JNK3的解离常数(Kd)为分别为22和76 nM,以及150和275 nM。分子建模表明在JNK催化位点的结合相互作用的模式,并且所选的肟衍生物是潜在的竞争性JNK抑制剂。化合物的JNK结合活性与其抑制脂多糖(LPS)诱导的人单核THP-1Blue细胞和白介素6(IL-β)中核因子-κB/活化蛋白1(NF-κB/ AP-1)活化的能力有关。 6)由人的MonoMac-6细胞产生。因此,具有茚并喹喔啉和色胺酮核的肟可以作为JNK机理研究的特定小分子调节剂,并可能成为开发消炎药的潜在先导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号