当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface‐Driven Keto–Enol Tautomerization: Atomistic Insights into Enol Formation and Stabilization Mechanisms
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-11-25 , DOI: 10.1002/anie.201808453
Smadar Attia 1, 2 , Marvin‐Christopher Schmidt 1 , Carsten Schröder 1 , Pascal Pessier 1 , Swetlana Schauermann 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-11-25 , DOI: 10.1002/anie.201808453
Smadar Attia 1, 2 , Marvin‐Christopher Schmidt 1 , Carsten Schröder 1 , Pascal Pessier 1 , Swetlana Schauermann 1
Affiliation
|
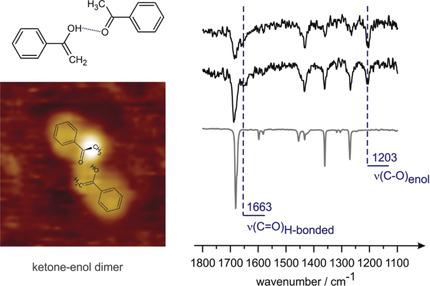
Tautomerisation of simple carbonyl compounds to their enol counterparts on metal surfaces is envisaged to enable an easier route for hydrogenation of the C=O bond in heterogeneously catalyzed reactions. To understand the mechanisms of enol formation and stabilization over catalytically active metal surfaces, we performed a mechanistic study on keto–enol tautomerization of a monocarbonyl compound acetophenon over Pt(111) surface. By employing infrared reflection adsorption spectroscopy in combination with scanning tunneling microscopy, we found that enol can be formed by building a ketone–enol dimer, in which one molecule in the enol form is stabilized through hydrogen bonding to the carbonyl group of the second ketone molecule. Based on the investigations of the co‐adsorption behavior of acetophenone and hydrogen, we conclude that keto–enol tautomerization occurs in the intramolecular process and does not involve hydrogen transfer through the surface hypothesized previously.
中文翻译:

表面驱动的酮-烯醇互变异构:烯醇形成和稳定机理的原子学见解
设想将简单的羰基化合物互变异构化为金属表面上的烯醇对应物,以使在异相催化反应中C = O键的氢化更容易。为了了解烯醇在催化活性金属表面上形成和稳定的机理,我们对Pt(111)表面单羰基化合物苯乙酮的酮-烯醇互变异构进行了机理研究。通过采用红外反射吸收光谱技术结合扫描隧道显微镜,我们发现可以通过构建酮-烯醇二聚体来形成烯醇,其中一个烯醇形式的分子通过氢键与第二个酮分子的羰基键合而得以稳定。根据对苯乙酮和氢的共吸附行为的研究,
更新日期:2018-11-25
中文翻译:

表面驱动的酮-烯醇互变异构:烯醇形成和稳定机理的原子学见解
设想将简单的羰基化合物互变异构化为金属表面上的烯醇对应物,以使在异相催化反应中C = O键的氢化更容易。为了了解烯醇在催化活性金属表面上形成和稳定的机理,我们对Pt(111)表面单羰基化合物苯乙酮的酮-烯醇互变异构进行了机理研究。通过采用红外反射吸收光谱技术结合扫描隧道显微镜,我们发现可以通过构建酮-烯醇二聚体来形成烯醇,其中一个烯醇形式的分子通过氢键与第二个酮分子的羰基键合而得以稳定。根据对苯乙酮和氢的共吸附行为的研究,






































 京公网安备 11010802027423号
京公网安备 11010802027423号