International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2018-10-11 , DOI: 10.1016/j.ijbiomac.2018.10.040 Nidhi Rani , Samannaya Hazra , Amrita Singh , Avadhesha Surolia
|
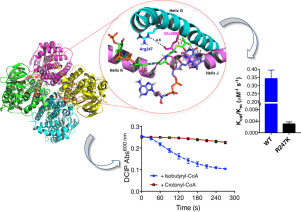
Members of the Acyl-CoA dehydrogenase (ACADs) family of enzymes play a crucial role in cholesterol and steroid catabolism and are widely studied in the oldest known human pathogen, Mycobacterium tuberculosis (Mtb). However, there is a paucity of information on ACADs involved in branched chain amino acid catabolism. Here we characterized one of the putative ACAD enzyme, fadE9, as “Isobutyryl CoA Dehydrogenase (IBDH)” using a combined computational and experimental approach, guided by homology modeled structural information, affirming its role in valine catabolism. Multiple sequence alignment and phylogenetic analysis place it in a separate cluster from a recently identified family of α2β2-heterotetramer ACADs in Mtb, based on the position of the conserved Arg247 and catalytic Glu368 residues. The conserved Arg247 was predicted to play an essential role at the center of H-bonding network of reaction center and was confirmed by the reduced activity of R247K mutant. Thus, in addition to the finding of an architecturally distinct α2β2-heterotetramer among ACADs, these studies also highlight the differences between MtIBDH, fadE9 from the other ACADs that are involved in cholesterol and steroid catabolism of Mtb.
中文翻译:

结核分枝杆菌推定时尚E9作为异丁酰辅酶A脱氢酶参与缬氨酸分解代谢的功能注释
酰基辅酶A脱氢酶(ACAD)家族的成员在胆固醇和类固醇的分解代谢中起着至关重要的作用,并且在已知最古老的人类病原体结核分枝杆菌(Mtb)中得到了广泛的研究。但是,关于与支链氨基酸分解代谢有关的ACAD的信息很少。在这里,我们采用组合的计算和实验方法,在同源性建模的结构信息的指导下,将一种推定的ACAD酶fad E9表征为“异丁酰CoA脱氢酶(IBDH)”,确认其在缬氨酸分解代谢中的作用。多重序列比对和系统发育分析将其与Mtb中最近鉴定的α2β2-异四聚体ACAD家族分开放置,基于保守的Arg247和催化性Glu368残基的位置。保守的Arg247被预测在反应中心的H键网络的中心发挥重要作用,并被R247K突变体的活性降低所证实。因此,除了在ACAD中发现结构上独特的α2β2-异四聚体外,这些研究还强调了Mt IBDH与fad E9与其他ACAD中涉及Mtb的胆固醇和类固醇分解代谢的差异。

































 京公网安备 11010802027423号
京公网安备 11010802027423号