当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydration Structures of Vanadium/Oxovanadium Cations in the Presence of Sulfuric Acid: A Molecular Dynamics Simulation Study
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.ces.2018.10.014 Ning Zhang , Boyun Yang , Jun Huo , Wenxu Qi , Xiaopeng Zhang , Xuehua Ruan , Junjiang Bao , Gaohong He
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.ces.2018.10.014 Ning Zhang , Boyun Yang , Jun Huo , Wenxu Qi , Xiaopeng Zhang , Xuehua Ruan , Junjiang Bao , Gaohong He
|
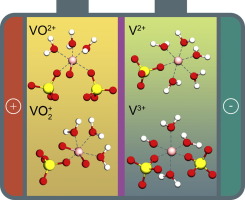
Abstract Structure design of proton exchange membrane determines the performance of vanadium redox flow battery. Appropriate pore size of the membrane not only benefits the proton conduction but also blocks the cross contamination of the vanadium cations. For this purpose, hydration sizes of proton, vanadium(II) [V2+], vanadium(III) [V3+], oxovanadium(IV) [VO2+], and dioxovanadium(V) [VO2+] cations were estimated by molecular dynamics simulation based on all-atom force field. The local hydration structures of the vanadium cations were consistent with the previous reports, verifying the adopted force field. V2+, V3+, VO2+, VO2+, and proton exhibit the hydration sizes of 8.24, 8.14, 8.18, 8.34, and 4.12 A in the aqueous mixtures of sulfuric acid and vanadium sulfate. Thus the pore size distribution from 4.12 to 8.14 A was recommended for the membrane fabrication. SO42− prefers to distribute around the vanadium cations due to electrostatic interaction, thus weakening the hydration structures of the vanadium cations. Introducing sulfuric acid decreases the local distributions of SO42− around V2+ and V3+, but increases the local distributions of SO42− around VO2+ and VO2+. This work helps to understand the structure of the aqueous mixtures of sulfuric acid and vanadium sulfate. It also provides potential guidance for improving the membrane performance in vanadium redox flow battery.
中文翻译:

硫酸存在下钒/氧钒阳离子的水合结构:分子动力学模拟研究
摘要 质子交换膜的结构设计决定了钒氧化还原液流电池的性能。合适的膜孔径不仅有利于质子传导,而且还能阻止钒阳离子的交叉污染。为此,通过分子动力学模拟估算了质子、钒 (II) [V2+]、钒 (III) [V3+]、氧钒 (IV) [VO2+] 和二氧钒 (V) [VO2+] 阳离子的水合尺寸全原子力场。钒阳离子的局部水合结构与之前的报告一致,验证了所采用的力场。V2+、V3+、VO2+、VO2+ 和质子在硫酸和硫酸钒的含水混合物中的水合尺寸分别为 8.24、8.14、8.18、8.34 和 4.12 A。因此孔径分布从 4.12 到 8。建议使用 14 A 进行膜制造。由于静电相互作用,SO42-更倾向于分布在钒阳离子周围,从而削弱了钒阳离子的水合结构。引入硫酸会降低 SO42− 在 V2+ 和 V3+ 周围的局部分布,但会增加 SO42− 在 VO2+ 和 VO2+ 周围的局部分布。这项工作有助于了解硫酸和硫酸钒的水性混合物的结构。它还为改善钒氧化还原液流电池的膜性能提供了潜在的指导。但增加了 SO42− 在 VO2+ 和 VO2+ 周围的局部分布。这项工作有助于了解硫酸和硫酸钒的水性混合物的结构。它还为改善钒氧化还原液流电池的膜性能提供了潜在的指导。但增加了 SO42− 在 VO2+ 和 VO2+ 周围的局部分布。这项工作有助于了解硫酸和硫酸钒的水性混合物的结构。它还为改善钒氧化还原液流电池的膜性能提供了潜在的指导。
更新日期:2019-02-01
中文翻译:

硫酸存在下钒/氧钒阳离子的水合结构:分子动力学模拟研究
摘要 质子交换膜的结构设计决定了钒氧化还原液流电池的性能。合适的膜孔径不仅有利于质子传导,而且还能阻止钒阳离子的交叉污染。为此,通过分子动力学模拟估算了质子、钒 (II) [V2+]、钒 (III) [V3+]、氧钒 (IV) [VO2+] 和二氧钒 (V) [VO2+] 阳离子的水合尺寸全原子力场。钒阳离子的局部水合结构与之前的报告一致,验证了所采用的力场。V2+、V3+、VO2+、VO2+ 和质子在硫酸和硫酸钒的含水混合物中的水合尺寸分别为 8.24、8.14、8.18、8.34 和 4.12 A。因此孔径分布从 4.12 到 8。建议使用 14 A 进行膜制造。由于静电相互作用,SO42-更倾向于分布在钒阳离子周围,从而削弱了钒阳离子的水合结构。引入硫酸会降低 SO42− 在 V2+ 和 V3+ 周围的局部分布,但会增加 SO42− 在 VO2+ 和 VO2+ 周围的局部分布。这项工作有助于了解硫酸和硫酸钒的水性混合物的结构。它还为改善钒氧化还原液流电池的膜性能提供了潜在的指导。但增加了 SO42− 在 VO2+ 和 VO2+ 周围的局部分布。这项工作有助于了解硫酸和硫酸钒的水性混合物的结构。它还为改善钒氧化还原液流电池的膜性能提供了潜在的指导。但增加了 SO42− 在 VO2+ 和 VO2+ 周围的局部分布。这项工作有助于了解硫酸和硫酸钒的水性混合物的结构。它还为改善钒氧化还原液流电池的膜性能提供了潜在的指导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号