Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Colonic Lysine Homocysteinylation Induced by High-Fat Diet Suppresses DNA Damage Repair.
Cell Reports ( IF 7.5 ) Pub Date : 2018-10-09 , DOI: 10.1016/j.celrep.2018.09.022 Dan Wang 1 , Rui Zhao 2 , Yuan-Yuan Qu 3 , Xin-Yu Mei 2 , Xuan Zhang 2 , Qian Zhou 2 , Yang Li 2 , Shao-Bo Yang 4 , Zhi-Gui Zuo 5 , Yi-Ming Chen 6 , Yan Lin 7 , Wei Xu 8 , Chao Chen 4 , Shi-Min Zhao 8 , Jian-Yuan Zhao 8
Cell Reports ( IF 7.5 ) Pub Date : 2018-10-09 , DOI: 10.1016/j.celrep.2018.09.022 Dan Wang 1 , Rui Zhao 2 , Yuan-Yuan Qu 3 , Xin-Yu Mei 2 , Xuan Zhang 2 , Qian Zhou 2 , Yang Li 2 , Shao-Bo Yang 4 , Zhi-Gui Zuo 5 , Yi-Ming Chen 6 , Yan Lin 7 , Wei Xu 8 , Chao Chen 4 , Shi-Min Zhao 8 , Jian-Yuan Zhao 8
Affiliation
|
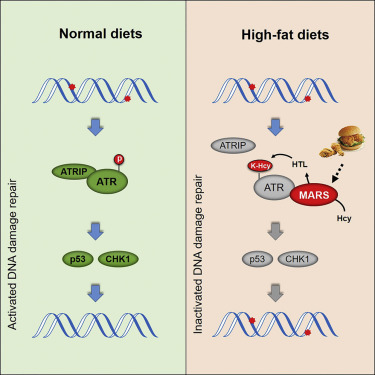
Colorectal cancer (CRC) onset is profoundly affected by Western diet. Here, we report that high-fat (HF) diet-induced, organ-specific colonic lysine homocysteinylation (K-Hcy) increase might promote CRC onset by impeding DNA damage repair. HF chow induced elevated methionyl-tRNA synthetase (MARS) expression and K-Hcy levels and DNA damage accumulation in the mouse and rat colon, resulting in a phenotype identical to that of CRC tissues. Moreover, the increased copy number of MARS, whose protein product promotes K-Hcy, correlated with increased CRC risk in humans. Mechanistically, MARS preferentially bound to and modified ataxia-telangiectasia and Rad3-related protein (ATR), inhibited ATR and its downstream effectors checkpoint kinase-1 and p53, and relieved cell-cycle arrest and decreased DNA damage-induced apoptosis by disrupting the binding of ATR-interacting protein to ATR. Inhibiting K-Hcy by targeting MARS reversed these effects and suppressed oncogenic CRC cell growth. Our study reveals a mechanism of Western-diet-associated CRC and highlights an intervention approach for reversing diet-induced oncogenic effects.
中文翻译:

高脂饮食引起的结肠赖氨酸同型半胱氨酸化抑制DNA损伤修复。
西方饮食严重影响了大肠癌的发病。在这里,我们报道高脂饮食诱导的器官特异性结肠赖氨酸高半胱氨酸化(K-Hcy)增加可能通过阻止DNA损伤修复而促进CRC的发作。HF引起的甲硫氨酰-tRNA合成酶(MARS)表达和K-Hcy水平升高,并且在小鼠和大鼠结肠中积累了DNA损伤,导致其表型与CRC组织的表型相同。此外,其蛋白质产物促进K-Hcy的MARS拷贝数增加与人类CRC风险增加相关。从机制上讲,MARS优先结合并修饰了共济失调-毛细血管扩张和Rad3相关蛋白(ATR),抑制了ATR及其下游效应子Checkpoint激酶1和p53,通过破坏ATR相互作用蛋白与ATR的结合,减轻细胞周期停滞并减少DNA损伤诱导的细胞凋亡。通过靶向MARS抑制K-Hcy可逆转这些作用并抑制致癌CRC细胞的生长。我们的研究揭示了与西方饮食相关的CRC的机制,并强调了一种逆转饮食引起的致癌作用的干预方法。
更新日期:2018-10-11
中文翻译:

高脂饮食引起的结肠赖氨酸同型半胱氨酸化抑制DNA损伤修复。
西方饮食严重影响了大肠癌的发病。在这里,我们报道高脂饮食诱导的器官特异性结肠赖氨酸高半胱氨酸化(K-Hcy)增加可能通过阻止DNA损伤修复而促进CRC的发作。HF引起的甲硫氨酰-tRNA合成酶(MARS)表达和K-Hcy水平升高,并且在小鼠和大鼠结肠中积累了DNA损伤,导致其表型与CRC组织的表型相同。此外,其蛋白质产物促进K-Hcy的MARS拷贝数增加与人类CRC风险增加相关。从机制上讲,MARS优先结合并修饰了共济失调-毛细血管扩张和Rad3相关蛋白(ATR),抑制了ATR及其下游效应子Checkpoint激酶1和p53,通过破坏ATR相互作用蛋白与ATR的结合,减轻细胞周期停滞并减少DNA损伤诱导的细胞凋亡。通过靶向MARS抑制K-Hcy可逆转这些作用并抑制致癌CRC细胞的生长。我们的研究揭示了与西方饮食相关的CRC的机制,并强调了一种逆转饮食引起的致癌作用的干预方法。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号