Tetrahedron ( IF 2.1 ) Pub Date : 2018-10-11 , DOI: 10.1016/j.tet.2018.10.016 Csilla Hargitai , Tamás Nagy , Judit Halász , Györgyi Koványi-Lax , Gábor Németh , Gyula Simig , Balázs Volk
|
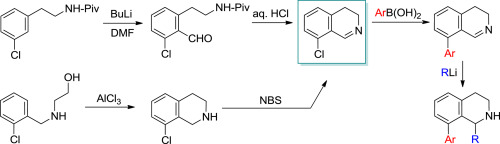
Two procedures for the synthesis of barely accessible 8-chloro-3,4-dihydroisoquinoline were investigated. The first approach is based on a directed ortho-lithiation of N-pivaloyl meta-chlorophenylethylamine, followed by formylation and subsequent ring closure under acidic conditions. In the second, more advantageous variant, the N-hydroxyethyl ortho-chlorobenzylamine intermediate undergoes a Friedel-Crafts reaction, and the resulting tetrahydro derivative is oxidized with N-bromosuccinimide. The 8-chloro-3,4-dihydroisoquinoline key intermediate is then applied in Suzuki reactions to give various 8-aryl-3,4-dihydroisoquinolines, which are finally treated with alkyl or aryllithiums to give 1-substituted 8-aryl-1,2,3,4-tetrahydroisoquinolines. These novel 1,2,3,4-tetrahydroisoquinoline derivatives can be used as building blocks in the synthesis of potential drug candidates.
中文翻译:

8-氯-3,4-二氢异喹啉的合成与进一步转化
研究了两种几乎无法获得的8-氯-3,4-二氢异喹啉的合成方法。第一种方法基于N-新戊酰基间-氯苯基乙胺的定向邻位锂化,然后进行甲酰化和随后在酸性条件下闭环。在第二个更有利的变体中,使N-羟乙基邻氯苄胺中间体进行弗里德-克来福特反应,并且将所得的四氢衍生物用N氧化。-溴代琥珀酰亚胺。然后将8-氯-3,4-二氢异喹啉关键中间体应用于Suzuki反应中,得到各种8-芳基-3,4-二氢异喹啉,最后用烷基或芳基锂处理得到1-取代的8-芳基-1, 2,3,4-四氢异喹啉。这些新颖的1,2,3,4-四氢异喹啉衍生物可用作潜在候选药物合成的基础。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号