Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2018-10-10 , DOI: 10.1016/j.snb.2018.10.043 Ying Hu , Joonyoung F. Joung , Ji-Eun Jeong , Yerin Jeong , Han Young Woo , Yuanbin She , Sungnam Park , Juyoung Yoon
|
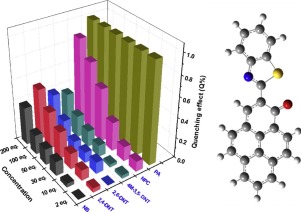
2-(Benzothiazol-2-yl)pyren-1-ol (P3-NS) was developed as a excited-state intramolecular proton transfer (ESIPT)- based sensor for nitroaromatic compounds (NACs). Results of studies of its photophysical and optical properties show that P3-NS exists in various, solvent dependent isomeric forms arising from rotation about the CC bond connecting the benzothiazole and pyren-1-ol rings and the location of the pyrene-OH proton. The results of density functional theory (DFT) calculations enabled identification of the major structures of P3-NS dissolved in different solvents. Upon electronic excitation in nonpolar or weakly polar solvents, P3-NS undergoes an ESIPT reaction to form a product that only weakly fluoresces. In contrast, P3-NS exists in a strongly fluorescent deprotonated form in highly polar solvents. The results of time-dependent DFT calculations indicate that fluorescence quenching of the product generated by ESIPT reaction of P3-NS is caused by intersystem crossing at a conical intersection between S1 and T2 states. The anionic form of P3-NS, present in highly polar solvents, was shown to be a fluorescence sensor for nitroaromatic compounds (NACs). Fluorescence quenching by NACs occurs by protonation of the anionic form of P3-NS (static quenching) and by photoinduced electron transfer from the anionic form to NACs (dynamic quenching).
中文翻译:

2-(Benzothiazol-2-yl)pyren-1-ol,一种新型的基于激发态分子内质子转移的硝基芳香族化合物荧光传感器
2-(Benzothiazol-2-yl)pyren-1-ol(P3-NS)被开发为基于激发态分子内质子转移(ESIPT)的硝基芳香族化合物(NAC)传感器。对它的光物理和光学性质的研究结果表明,P3-NS以各种溶剂依赖性异构体形式存在,这些异构体是围绕围绕苯并噻唑和and-1-醇环的C C键旋转以及the-OH质子的位置而产生的。密度泛函理论(DFT)计算的结果能够识别溶解在不同溶剂中的P3-NS的主要结构。在非极性或弱极性溶剂中电子激发时,P3-NS经历ESIPT反应形成仅微弱发荧光的产物。相反,P3-NS在强极性溶剂中以强荧光去质子化形式存在。随时间变化的DFT计算结果表明,由P3-NS的ESIPT反应生成的产物的荧光猝灭是由在S 1和T 2状态之间的圆锥形交叉处的系统间交叉引起的。存在于高极性溶剂中的P3-NS阴离子形式显示为硝基芳香族化合物(NAC)的荧光传感器。NAC的荧光猝灭是通过P3-NS阴离子形式的质子化而发生的 (静态猝灭)和通过光诱导电子从阴离子形式转移到NAC(动态猝灭)。































 京公网安备 11010802027423号
京公网安备 11010802027423号