当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Pyrrolidine Sulfonamides as Selective and Orally Bioavailable Antagonists of Transient Receptor Potential Vanilloid-4 (TRPV4)
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01317 Edward J. Brnardic , Guosen Ye , Carl Brooks , Carla Donatelli , Linda Barton , Jeff McAtee , Robert M. Sanchez , Arthur Shu , Karl Erhard , Lamont Terrell , Grazyna Graczyk-Millbrandt , Yanan He , Melissa H. Costell , David J. Behm , Theresa Roethke , Patrick Stoy , Dennis A. Holt , Brian G. Lawhorn
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01317 Edward J. Brnardic , Guosen Ye , Carl Brooks , Carla Donatelli , Linda Barton , Jeff McAtee , Robert M. Sanchez , Arthur Shu , Karl Erhard , Lamont Terrell , Grazyna Graczyk-Millbrandt , Yanan He , Melissa H. Costell , David J. Behm , Theresa Roethke , Patrick Stoy , Dennis A. Holt , Brian G. Lawhorn
|
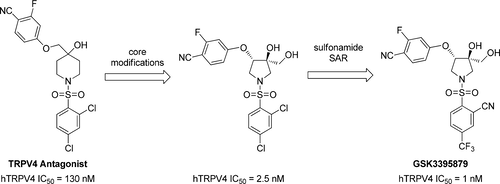
A novel series of pyrrolidine sulfonamide transient receptor potential vanilloid-4 (TRPV4) antagonists was developed by modification of a previously reported TRPV4 inhibitor (1). Several core-structure modifications were identified that improved TRPV4 activity by increasing structural rigidity and reducing the entropic energy penalty upon binding to the target protein. The new template was initially discovered as a minor regio-isomeric side product formed during routine structure–activity relationship (SAR) studies, and further optimization resulted in highly potent compounds with a novel pyrrolidine diol core. Further improvements in potency and pharmacokinetic properties were achieved through SAR studies on the sulfonamide substituent to give an optimized lead compound GSK3395879 (52) that demonstrated the ability to inhibit TRPV4-mediated pulmonary edema in an in vivo rat model. GSK3395879 is a tool for studying the biology of TRPV4 and an advanced lead for identifying new heart failure medicines.
中文翻译:

吡咯烷磺酰胺作为瞬时受体潜在Vanilloid-4(TRPV4)的选择性和口服生物利用拮抗剂的发现。
通过修饰先前报道的TRPV4抑制剂(1),开发了一系列新型的吡咯烷磺酰胺瞬时受体电位香草酸4(TRPV4)拮抗剂。鉴定了几种核心结构修饰,它们通过增加结构刚度和减少与靶蛋白结合后的熵能损失而改善了TRPV4的活性。新模板最初是在常规的结构-活性关系(SAR)研究期间发现的,是次要的区域异构副产物,进一步的优化导致了具有新型吡咯烷二醇核心的高效化合物。通过对磺酰胺取代基进行SAR研究,进一步提高了药效和药代动力学性能,从而获得了优化的铅化合物GSK3395879(52)表明在体内大鼠模型中具有抑制TRPV4介导的肺水肿的能力。GSK3395879是研究TRPV4生物学的工具,也是鉴定新的心力衰竭药物的先进线索。
更新日期:2018-10-10
中文翻译:

吡咯烷磺酰胺作为瞬时受体潜在Vanilloid-4(TRPV4)的选择性和口服生物利用拮抗剂的发现。
通过修饰先前报道的TRPV4抑制剂(1),开发了一系列新型的吡咯烷磺酰胺瞬时受体电位香草酸4(TRPV4)拮抗剂。鉴定了几种核心结构修饰,它们通过增加结构刚度和减少与靶蛋白结合后的熵能损失而改善了TRPV4的活性。新模板最初是在常规的结构-活性关系(SAR)研究期间发现的,是次要的区域异构副产物,进一步的优化导致了具有新型吡咯烷二醇核心的高效化合物。通过对磺酰胺取代基进行SAR研究,进一步提高了药效和药代动力学性能,从而获得了优化的铅化合物GSK3395879(52)表明在体内大鼠模型中具有抑制TRPV4介导的肺水肿的能力。GSK3395879是研究TRPV4生物学的工具,也是鉴定新的心力衰竭药物的先进线索。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号