Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CX3CR1 differentiates F4/80low monocytes into pro-inflammatory F4/80high macrophages in the liver.
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Oct-10 , DOI: 10.1038/s41598-018-33440-9 Young-Sun Lee , Myung-Ho Kim , Hyon-Seung Yi , So Yeon Kim , Hee-Hoon Kim , Ji Hoon Kim , Jong Eun Yeon , Kwan Soo Byun , Jin-Seok Byun , Won-Il Jeong
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Oct-10 , DOI: 10.1038/s41598-018-33440-9 Young-Sun Lee , Myung-Ho Kim , Hyon-Seung Yi , So Yeon Kim , Hee-Hoon Kim , Ji Hoon Kim , Jong Eun Yeon , Kwan Soo Byun , Jin-Seok Byun , Won-Il Jeong
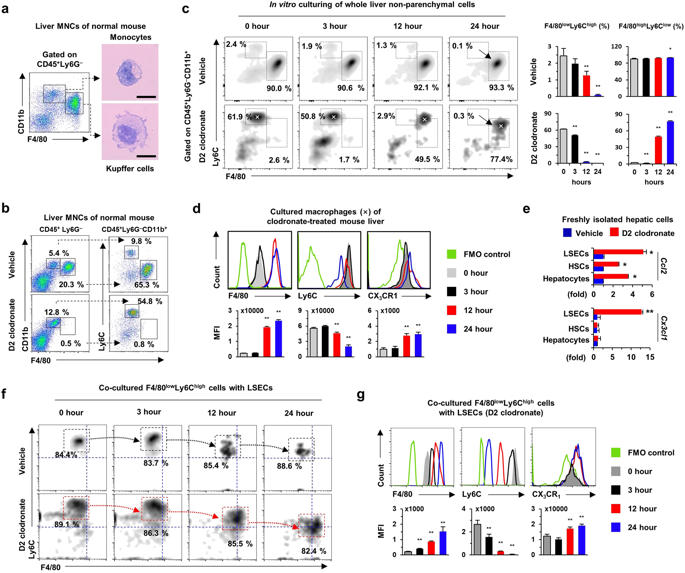
|
The expression of chemokine receptor CX3CR1 is related to migration and signaling in cells of the monocyte-macrophage lineage. The precise roles of CX3CR1 in the liver have been investigated but not clearly elucidated. Here, we investigated the roles of CX3CR1 in hepatic macrophages and liver injury. Hepatic and splenic CX3CR1lowF4/80low monocytes and CX3CR1lowCD16- monocytes were differentiated into CX3CR1highF4/80high or CX3CR1highCD16+ macrophages by co-culture with endothelial cells. Moreover, CX3CL1 deficiency in human umbilical vein endothelial cells (HUVECs) attenuated the expression of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), whereas recombinant CX3CL1 treatment reversed this expression in co-cultured monocytes. Upon treatment with clodronate liposome, hepatic F4/80high macrophages were successfully depleted at day 2 and recovered similarly in CX3CR1+/GFP and CX3CR1GFP/GFP mice at week 4, suggesting a CX3CR1-independent replacement. However, F4/80high macrophages of CX3CR1+/GFP showed a stronger pro-inflammatory phenotype than CX3CR1GFP/GFP mice. In clodronate-treated chimeric CX3CR1+/GFP and CX3CR1GFP/GFP mice, CX3CR1+F4/80high macrophages showed higher expression of IL-1β and TNF-α than CX3CR1-F4/80high macrophages. In alcoholic liver injury, despite the similar frequency of hepatic F4/80high macrophages, CX3CR1GFP/GFP mice showed reduced liver injury, hepatic fat accumulation, and inflammatory responses than CX3CR1+/GFP mice. Thus, CX3CR1 could be a novel therapeutic target for pro-inflammatory macrophage-mediated liver injury.
中文翻译:

CX3CR1在肝脏中将F4 / 80低单核细胞分化为促炎性F4 / 80高巨噬细胞。
趋化因子受体CX 3 CR1的表达与单核巨噬细胞谱系细胞中的迁移和信号传导有关。已经研究了CX 3 CR1在肝脏中的确切作用,但尚未明确阐明。在这里,我们调查了CX 3 CR1在肝巨噬细胞和肝损伤中的作用。肝和脾CX 3 CR1低F4 / 80低的单核细胞和CX 3 CR1低CD16 -单核细胞分化为CX 3 CR1高F4 / 80高或CX 3 CR1高CD16 +巨噬细胞与内皮细胞共培养。此外,人脐静脉内皮细胞(HUVECs)中CX 3 CL1的缺乏减弱了白介素1β(IL-1β)和肿瘤坏死因子-α(TNF-α)的表达,而重组CX 3 CL1处理逆转了这种表达。培养的单核细胞。用氯膦酸盐脂质体处理后,肝F4 / 80高巨噬细胞在第2天成功耗尽,并在第4周在CX 3 CR1 + / GFP和CX 3 CR1 GFP / GFP小鼠中类似地恢复,表明是CX 3 CR1独立的替代。但是,CX 3 CR1的F4 / 80高巨噬细胞+ / GFP显示出比CX 3 CR1 GFP / GFP小鼠更强的促炎表型。在用氯膦酸盐处理过的嵌合CX 3 CR1 + / GFP和CX 3 CR1 GFP / GFP小鼠中,CX 3 CR1 + F4 / 80高巨噬细胞的IL-1β和TNF-α表达高于CX 3 CR1 - F4 / 80高巨噬细胞。在酒精性肝损伤中,尽管肝F4 / 80高巨噬细胞的发生频率相似,但CX 3 CR1 GFP / GFP小鼠显示出比CX 3 CR1 + / GFP小鼠减少的肝损伤,肝脂肪蓄积和炎症反应。因此,CX 3 CR1可能是促炎性巨噬细胞介导的肝损伤的新型治疗靶点。
更新日期:2018-10-10
中文翻译:

CX3CR1在肝脏中将F4 / 80低单核细胞分化为促炎性F4 / 80高巨噬细胞。
趋化因子受体CX 3 CR1的表达与单核巨噬细胞谱系细胞中的迁移和信号传导有关。已经研究了CX 3 CR1在肝脏中的确切作用,但尚未明确阐明。在这里,我们调查了CX 3 CR1在肝巨噬细胞和肝损伤中的作用。肝和脾CX 3 CR1低F4 / 80低的单核细胞和CX 3 CR1低CD16 -单核细胞分化为CX 3 CR1高F4 / 80高或CX 3 CR1高CD16 +巨噬细胞与内皮细胞共培养。此外,人脐静脉内皮细胞(HUVECs)中CX 3 CL1的缺乏减弱了白介素1β(IL-1β)和肿瘤坏死因子-α(TNF-α)的表达,而重组CX 3 CL1处理逆转了这种表达。培养的单核细胞。用氯膦酸盐脂质体处理后,肝F4 / 80高巨噬细胞在第2天成功耗尽,并在第4周在CX 3 CR1 + / GFP和CX 3 CR1 GFP / GFP小鼠中类似地恢复,表明是CX 3 CR1独立的替代。但是,CX 3 CR1的F4 / 80高巨噬细胞+ / GFP显示出比CX 3 CR1 GFP / GFP小鼠更强的促炎表型。在用氯膦酸盐处理过的嵌合CX 3 CR1 + / GFP和CX 3 CR1 GFP / GFP小鼠中,CX 3 CR1 + F4 / 80高巨噬细胞的IL-1β和TNF-α表达高于CX 3 CR1 - F4 / 80高巨噬细胞。在酒精性肝损伤中,尽管肝F4 / 80高巨噬细胞的发生频率相似,但CX 3 CR1 GFP / GFP小鼠显示出比CX 3 CR1 + / GFP小鼠减少的肝损伤,肝脂肪蓄积和炎症反应。因此,CX 3 CR1可能是促炎性巨噬细胞介导的肝损伤的新型治疗靶点。































 京公网安备 11010802027423号
京公网安备 11010802027423号