Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Graphene Oxide-Gold Nanosheets Containing Chitosan Scaffold Improves Ventricular Contractility and Function After Implantation into Infarcted Heart.
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Oct-10 , DOI: 10.1038/s41598-018-33144-0 Sekaran Saravanan , Niketa Sareen , Ejlal Abu-El-Rub , Hend Ashour , Glen Lester Sequiera , Hania I. Ammar , Venkatraman Gopinath , Ashraf Ali Shamaa , Safinaz Salah Eldin Sayed , Meenal Moudgil , Jamuna Vadivelu , Sanjiv Dhingra
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Oct-10 , DOI: 10.1038/s41598-018-33144-0 Sekaran Saravanan , Niketa Sareen , Ejlal Abu-El-Rub , Hend Ashour , Glen Lester Sequiera , Hania I. Ammar , Venkatraman Gopinath , Ashraf Ali Shamaa , Safinaz Salah Eldin Sayed , Meenal Moudgil , Jamuna Vadivelu , Sanjiv Dhingra
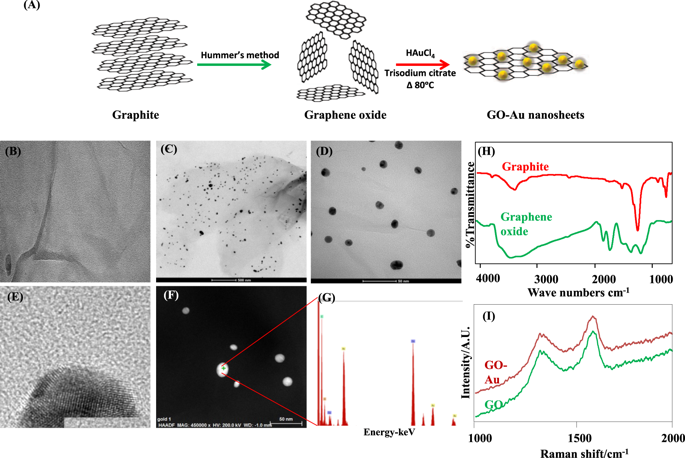
|
Abnormal conduction and improper electrical impulse propagation are common in heart after myocardial infarction (MI). The scar tissue is non-conductive therefore the electrical communication between adjacent cardiomyocytes is disrupted. In the current study, we synthesized and characterized a conductive biodegradable scaffold by incorporating graphene oxide gold nanosheets (GO-Au) into a clinically approved natural polymer chitosan (CS). Inclusion of GO-Au nanosheets in CS scaffold displayed two fold increase in electrical conductivity. The scaffold exhibited excellent porous architecture with desired swelling and controlled degradation properties. It also supported cell attachment and growth with no signs of discrete cytotoxicity. In a rat model of MI, in vivo as well as in isolated heart, the scaffold after 5 weeks of implantation showed a significant improvement in QRS interval which was associated with enhanced conduction velocity and contractility in the infarct zone by increasing connexin 43 levels. These results corroborate that implantation of novel conductive polymeric scaffold in the infarcted heart improved the cardiac contractility and restored ventricular function. Therefore, our approach may be useful in planning future strategies to construct clinically relevant conductive polymer patches for cardiac patients with conduction defects.
中文翻译:

包含壳聚糖支架的氧化石墨烯-金纳米片改善了植入梗塞心脏后的心室收缩力和功能。
心肌梗死(MI)后,心脏中异常传导和电脉冲传播不当的现象很普遍。疤痕组织是不导电的,因此相邻心肌细胞之间的电连通被破坏。在当前的研究中,我们通过将氧化石墨烯金纳米片(GO-Au)掺入临床批准的天然聚合物壳聚糖(CS)中来合成和表征导电可生物降解的支架。CS支架中包含GO-Au纳米片显示出两倍的电导率增加。该支架表现出优异的多孔结构,具有所需的溶胀和受控的降解性能。它也支持细胞附着和生长,没有离散的细胞毒性迹象。在心肌梗死的大鼠模型中,无论是体内还是离体心脏,植入5周后,支架显示QRS间隔显着改善,这与通过增加连接蛋白43水平而提高了梗死区的传导速度和收缩能力有关。这些结果证实了在梗塞的心脏中新型导电聚合物支架的植入改善了心脏的收缩力并恢复了心室功能。因此,我们的方法可能有助于规划未来策略,为患有传导缺陷的心脏病患者构建临床相关的导电聚合物贴剂。这些结果证实了在梗塞的心脏中新型导电聚合物支架的植入改善了心脏的收缩力并恢复了心室功能。因此,我们的方法可能有助于规划未来策略,为患有传导缺陷的心脏病患者构建临床相关的导电聚合物贴剂。这些结果证实了在梗塞的心脏中新型导电聚合物支架的植入改善了心脏的收缩力并恢复了心室功能。因此,我们的方法可能有助于规划未来策略,为患有传导缺陷的心脏病患者构建临床相关的导电聚合物贴剂。
更新日期:2018-10-10
中文翻译:

包含壳聚糖支架的氧化石墨烯-金纳米片改善了植入梗塞心脏后的心室收缩力和功能。
心肌梗死(MI)后,心脏中异常传导和电脉冲传播不当的现象很普遍。疤痕组织是不导电的,因此相邻心肌细胞之间的电连通被破坏。在当前的研究中,我们通过将氧化石墨烯金纳米片(GO-Au)掺入临床批准的天然聚合物壳聚糖(CS)中来合成和表征导电可生物降解的支架。CS支架中包含GO-Au纳米片显示出两倍的电导率增加。该支架表现出优异的多孔结构,具有所需的溶胀和受控的降解性能。它也支持细胞附着和生长,没有离散的细胞毒性迹象。在心肌梗死的大鼠模型中,无论是体内还是离体心脏,植入5周后,支架显示QRS间隔显着改善,这与通过增加连接蛋白43水平而提高了梗死区的传导速度和收缩能力有关。这些结果证实了在梗塞的心脏中新型导电聚合物支架的植入改善了心脏的收缩力并恢复了心室功能。因此,我们的方法可能有助于规划未来策略,为患有传导缺陷的心脏病患者构建临床相关的导电聚合物贴剂。这些结果证实了在梗塞的心脏中新型导电聚合物支架的植入改善了心脏的收缩力并恢复了心室功能。因此,我们的方法可能有助于规划未来策略,为患有传导缺陷的心脏病患者构建临床相关的导电聚合物贴剂。这些结果证实了在梗塞的心脏中新型导电聚合物支架的植入改善了心脏的收缩力并恢复了心室功能。因此,我们的方法可能有助于规划未来策略,为患有传导缺陷的心脏病患者构建临床相关的导电聚合物贴剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号