当前位置:
X-MOL 学术
›
J. Polym. Sci. A Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of asymmetrically substituted head‐to‐head polyacetylenes from 2,3‐disubstituted‐1,3‐butadienes
Journal of Polymer Science Part A: Polymer Chemistry Pub Date : 2018-10-09 , DOI: 10.1002/pola.29215 Yunhai Yu 1 , Chengke Qu 1 , Junpo He 1
Journal of Polymer Science Part A: Polymer Chemistry Pub Date : 2018-10-09 , DOI: 10.1002/pola.29215 Yunhai Yu 1 , Chengke Qu 1 , Junpo He 1
Affiliation
|
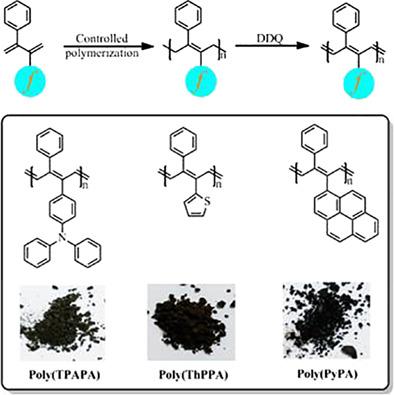
Asymmetrically substituted head‐to‐head polyacetylenes with phenyl and triphenylamine, thienyl or pyrenyl side groups were synthesized through anionic or controlled radical polymerization of 2,3‐disubstituted‐1,3‐butadienes and subsequent dehydrogenation process. Anionic polymerizations of the designed monomers bearing pendent triphenylamine and thienyl group gave narrow disperse disubstituted precursor polybutadienes with exclusive 1,4‐ or 4,1‐structure, which were confirmed by GPC and NMR measurements. In addition, the monomers possessing pyrenyl group were polymerized via nitroxide mediated radical polymerization and the resulting polymers were obtained with controlled molecular weight and low polydispersities. These polybutadiene precursors were then dehydrogenated in the presence of 2,3‐dichloro‐5,6‐dicyano‐1,4‐benzoquinone. Thus asymmetrically substituted head‐to‐head polyacetylenes were obtained as indicated by 1H NMR. The properties of polybutadiene precursors and the corresponding polyacetylenes were analyzed by UV–vis, DSC, and TGA. © 2018 Wiley Periodicals, Inc. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 395–402
中文翻译:

由2,3-二取代-1,3-丁二烯合成不对称取代的头对头聚乙炔
通过2,3-二取代-1,3-丁二烯的阴离子或受控自由基聚合以及随后的脱氢过程合成了具有苯基和三苯胺,噻吩基或pyr苯基侧基的不对称取代的头对头聚乙炔。设计的带有悬垂的三苯胺和噻吩基的单体的阴离子聚合反应得到窄分散的双取代前体聚丁二烯,具有独有的1,4-或4,1-结构,这已通过GPC和NMR测量得到证实。另外,通过氮氧化物介导的自由基聚合使具有pyr基的单体聚合,得到的聚合物具有受控的分子量和低的多分散性。然后将这些聚丁二烯前体在2,3-二氯-5,6-二氰基-1,4-苯醌存在下进行脱氢。1 H NMR。通过UV-vis,DSC和TGA分析了聚丁二烯前体和相应的聚乙炔的性质。©2018 Wiley Periodicals,Inc.J.Polym。科学,A部分:Polym。化学 2019,57,395-402
更新日期:2018-10-09
中文翻译:

由2,3-二取代-1,3-丁二烯合成不对称取代的头对头聚乙炔
通过2,3-二取代-1,3-丁二烯的阴离子或受控自由基聚合以及随后的脱氢过程合成了具有苯基和三苯胺,噻吩基或pyr苯基侧基的不对称取代的头对头聚乙炔。设计的带有悬垂的三苯胺和噻吩基的单体的阴离子聚合反应得到窄分散的双取代前体聚丁二烯,具有独有的1,4-或4,1-结构,这已通过GPC和NMR测量得到证实。另外,通过氮氧化物介导的自由基聚合使具有pyr基的单体聚合,得到的聚合物具有受控的分子量和低的多分散性。然后将这些聚丁二烯前体在2,3-二氯-5,6-二氰基-1,4-苯醌存在下进行脱氢。1 H NMR。通过UV-vis,DSC和TGA分析了聚丁二烯前体和相应的聚乙炔的性质。©2018 Wiley Periodicals,Inc.J.Polym。科学,A部分:Polym。化学 2019,57,395-402













































 京公网安备 11010802027423号
京公网安备 11010802027423号