当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight on the Formation of GaN:ZnO Solid Solution from Zn-Ga Layered Double Hydroxide Using Urea as the Nitriding Agent
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-10-08 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02498 Kiyofumi Katagiri 1 , Yuki Hayashi 1 , Risa Yoshiyuki 1 , Kei Inumaru 1 , Tomoki Uchiyama 2 , Noriyuki Nagata 2 , Yoshiharu Uchimoto 2 , Akinobu Miyoshi 3 , Kazuhiko Maeda 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-10-08 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02498 Kiyofumi Katagiri 1 , Yuki Hayashi 1 , Risa Yoshiyuki 1 , Kei Inumaru 1 , Tomoki Uchiyama 2 , Noriyuki Nagata 2 , Yoshiharu Uchimoto 2 , Akinobu Miyoshi 3 , Kazuhiko Maeda 3
Affiliation
|
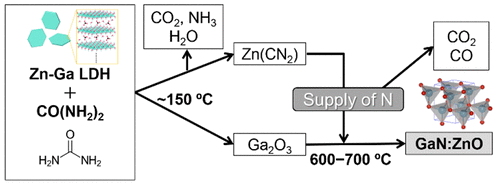
A solid solution of GaN and ZnO (GaN:ZnO) is promising as a photocatalyst for visible-light-driven overall water splitting to produce H2. However, several obstacles still exist in the conventional preparation procedure of GaN:ZnO. For example, the atomic distributions of Zn and Ga are nonuniform in GaN:ZnO when a mixture of the metal oxides, i.e. Ga2O3 and ZnO, is used as a precursor. In addition, GaN:ZnO is generally prepared under a harmful NH3 flow for long durations at high temperatures. Here, a facile synthesis of GaN:ZnO with homogeneous atomic composition via a simple and safe procedure is reported. A layered double hydroxide (LDH) containing Zn2+ and Ga3+ was used to increase the uniformity of the atomic distributions of Zn and Ga in GaN:ZnO. We employed urea as a nitriding agent instead of gaseous NH3 to increase the safety of the reaction. Through the optimization of reaction conditions such as heat treatment temperature and content of urea, single-phase GaN:ZnO was successfully obtained. In addition, the nitridation mechanism using urea was investigated in detail. NH3 released from the thermal decomposition of urea did not directly nitride the LDH precursor. X-ray absorption and infrared spectroscopies revealed that Zn(CN2)-like intermediate species were generated at the middle temperature range and Ga–N bonds formed at high temperature along with dissociation of CO and CO2.
中文翻译:

尿素为氮化剂的Zn-Ga层状双氢氧化物形成GaN:ZnO固溶体的机理研究
GaN和ZnO的固溶体(GaN:ZnO)有望作为光催化剂用于可见光驱动的总水分解产生H 2。然而,在常规的GaN:ZnO制备过程中仍然存在一些障碍。例如,当金属氧化物即Ga 2 O 3和ZnO的混合物用作前体时,Zn:和Ga的原子分布在GaN:ZnO中是不均匀的。此外,通常在高温下长时间在有害NH 3流下制备GaN:ZnO 。在此,报道了通过简单且安全的程序容易地合成具有均一原子组成的GaN:ZnO。含Zn 2+和Ga 3+的层状双氢氧化物(LDH)用于提高GaN:ZnO中Zn和Ga原子分布的均匀性。我们使用尿素代替气态NH 3作为氮化剂,以提高反应的安全性。通过优化热处理温度和尿素含量等反应条件,成功获得了单相GaN:ZnO。另外,详细研究了使用尿素的氮化机理。从尿素的热分解释放的NH 3不会直接氮化LDH前体。X射线吸收和红外光谱分析表明,在中等温度范围内会生成类似Zn(CN 2)的中间物种,并且在高温下会随着CO和CO 2的解离形成Ga-N键。
更新日期:2018-10-08
中文翻译:

尿素为氮化剂的Zn-Ga层状双氢氧化物形成GaN:ZnO固溶体的机理研究
GaN和ZnO的固溶体(GaN:ZnO)有望作为光催化剂用于可见光驱动的总水分解产生H 2。然而,在常规的GaN:ZnO制备过程中仍然存在一些障碍。例如,当金属氧化物即Ga 2 O 3和ZnO的混合物用作前体时,Zn:和Ga的原子分布在GaN:ZnO中是不均匀的。此外,通常在高温下长时间在有害NH 3流下制备GaN:ZnO 。在此,报道了通过简单且安全的程序容易地合成具有均一原子组成的GaN:ZnO。含Zn 2+和Ga 3+的层状双氢氧化物(LDH)用于提高GaN:ZnO中Zn和Ga原子分布的均匀性。我们使用尿素代替气态NH 3作为氮化剂,以提高反应的安全性。通过优化热处理温度和尿素含量等反应条件,成功获得了单相GaN:ZnO。另外,详细研究了使用尿素的氮化机理。从尿素的热分解释放的NH 3不会直接氮化LDH前体。X射线吸收和红外光谱分析表明,在中等温度范围内会生成类似Zn(CN 2)的中间物种,并且在高温下会随着CO和CO 2的解离形成Ga-N键。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号