Vaccine ( IF 4.5 ) Pub Date : 2018-10-09 , DOI: 10.1016/j.vaccine.2018.09.026 Lina Odevall , Deborah Hong , Laura Digilio , Sushant Sahastrabuddhe , Vittal Mogasale , Yeongok Baik , Seukkeun Choi , Jerome H. Kim , Julia Lynch
|
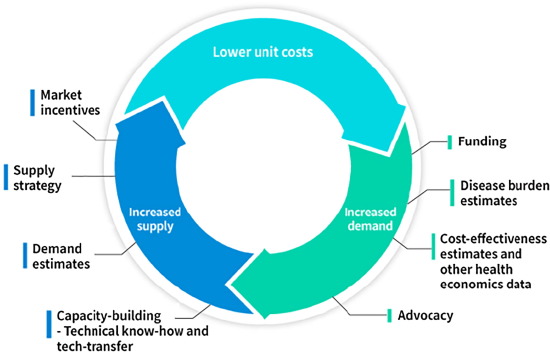
Cholera, a diarrheal disease primarily affecting vulnerable populations in developing countries, is estimated to cause disease in more than 2.5 million people and kill almost 100,000 annually. An oral cholera vaccine (OCV) has been available globally since 2001; the demand for this vaccine from affected countries has however been very low, due to various factors including vaccine price and mode of administration. The low demand for the vaccine and limited commercial incentives to invest in research and development of vaccines for developing country markets has kept the global supply of OCVs down. Since 1999, the International Vaccine Institute has been committed to make safe, effective and affordable OCVs accessible. Through a variety of partnerships with collaborators in Sweden, Vietnam, India and South Korea, and with public and private funding, IVI facilitated development and production of two affordable and WHO-prequalified OCVs and together with other stakeholders accelerated the introduction of these vaccines for the global public-sector market.






























 京公网安备 11010802027423号
京公网安备 11010802027423号