当前位置:
X-MOL 学术
›
Appl. Catal. A Gen.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective hydrophosphonylation of N- benzyl imines, isatin derived ketimines and isatins catalyzed by in-situ generated Ti(IV) macrocyclic salen complexes
Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2016-02-14 13:31:49
Mohd Nazish, Ajay Jakhar, Noor-ul H. Khan, Shailesh Verma, Rukhsana I. Kureshy, Sayed H.R. Abdi, Hari C. Bajaj
Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2016-02-14 13:31:49
Mohd Nazish, Ajay Jakhar, Noor-ul H. Khan, Shailesh Verma, Rukhsana I. Kureshy, Sayed H.R. Abdi, Hari C. Bajaj
|
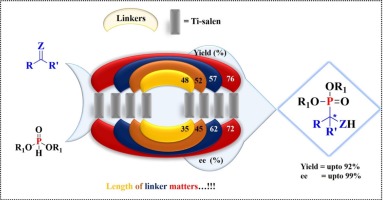
Ti-salen complexes were generated by using a series of chiral macrocyclic salen ligands and were used as catalysts for enantioselective hydrophosphonylation (EHP) reaction of benzylimines, isatin derived ketimines and isatins. The corresponding phosphonylated products were obtained with excellent yield (up to 92%) and enantioselectivity (ee up to 99%) with low catalyst loading at room temperature using dimethyl phosphite as nucleophile (IIa) for isatins and benzylimines, whereas for ketimines diphenyl phosphite (IIb) gave best results with very good yield (up to 88%) and ee (up to 99%). The Ti(IV) complex was recoverable and recyclable with retention of its catalytic performance at gram scale level. To understand the reaction mechanism NMR studies have been carried out using benzylimine as a model substrate and dimethyl phosphite as a nucleophile.
中文翻译:

原位生成的Ti(IV)大环Salen配合物催化N-苄基亚胺,Isatin衍生的酮亚胺和isatins的对映选择性氢膦酰基化
Ti-salen配合物是通过使用一系列手性大环塞伦配体生成的,并用作苄亚胺,靛红衍生的酮亚胺和靛红的对映选择性氢膦酰化(EHP)反应的催化剂。使用亚磷酸二甲酯作为亲核试剂(IIa),对于靛红和苄基亚胺,而对于酮亚胺二亚磷酸亚砜( IIb)以极好的收率(高达88%)和ee(高达99%)给出了最佳结果。Ti(IV)配合物可回收并可回收,并且其催化性能保持在克级。为了理解反应机理,已经使用苄基亚胺作为模型底物和亚磷酸二甲酯作为亲核试剂进行了NMR研究。
更新日期:2016-02-15
中文翻译:

原位生成的Ti(IV)大环Salen配合物催化N-苄基亚胺,Isatin衍生的酮亚胺和isatins的对映选择性氢膦酰基化
Ti-salen配合物是通过使用一系列手性大环塞伦配体生成的,并用作苄亚胺,靛红衍生的酮亚胺和靛红的对映选择性氢膦酰化(EHP)反应的催化剂。使用亚磷酸二甲酯作为亲核试剂(IIa),对于靛红和苄基亚胺,而对于酮亚胺二亚磷酸亚砜( IIb)以极好的收率(高达88%)和ee(高达99%)给出了最佳结果。Ti(IV)配合物可回收并可回收,并且其催化性能保持在克级。为了理解反应机理,已经使用苄基亚胺作为模型底物和亚磷酸二甲酯作为亲核试剂进行了NMR研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号