当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Characterization of a Thioesterase-Specific Monoclonal Antibody That Recognizes the 6-Deoxyerythronolide B Synthase
Biochemistry ( IF 2.9 ) Pub Date : 2018-10-05 00:00:00 , DOI: 10.1021/acs.biochem.8b00886 Xiuyuan Li , Natalia Sevillano 1 , Florencia La Greca 1 , Jake Hsu , Irimpan I. Mathews 2 , Tsutomu Matsui 1 , Charles S. Craik 1 , Chaitan Khosla
Biochemistry ( IF 2.9 ) Pub Date : 2018-10-05 00:00:00 , DOI: 10.1021/acs.biochem.8b00886 Xiuyuan Li , Natalia Sevillano 1 , Florencia La Greca 1 , Jake Hsu , Irimpan I. Mathews 2 , Tsutomu Matsui 1 , Charles S. Craik 1 , Chaitan Khosla
Affiliation
|
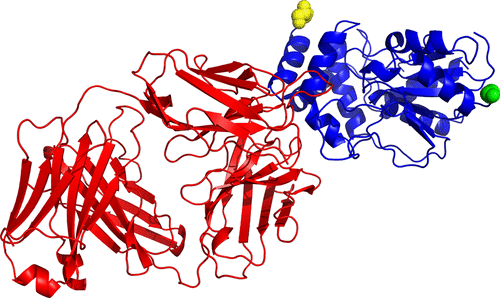
Assembly line polyketide synthases (PKSs) are large multimodular enzymes responsible for the biosynthesis of diverse antibiotics in bacteria. Structural and mechanistic analysis of these megasynthases can benefit from the discovery of reagents that recognize individual domains or linkers in a site-specific manner. Monoclonal antibodies not only have proven themselves as premier tools in analogous applications but also have the added benefit of constraining the conformational flexibility of their targets in unpredictable but often useful ways. Here we have exploited a library based on the naïve human antibody repertoire to discover a Fab (3A6) that recognizes the terminal thioesterase (TE) domain of the 6-deoxyerythronolide B synthase with high specificity. Biochemical assays were used to verify that 3A6 binding does not inhibit enzyme turnover. The co-crystal structure of the TE–3A6 complex was determined at 2.45 Å resolution, resulting in atomic characterization of this protein–protein recognition mechanism. Fab binding had minimal effects on the structural integrity of the TE. In turn, these insights were used to interrogate via small-angle X-ray scattering the solution-phase conformation of 3A6 complexed to a catalytically competent PKS module and bimodule. Altogether, we have developed a high-affinity monoclonal antibody tool that recognizes the TE domain of the 6-deoxyerythronolide B synthase while maintaining its native function.
中文翻译:

发现和表征的硫酯酶特异性单克隆抗体识别6-Deoxyerythronolide B合酶。
组装线聚酮化合物合酶(PKSs)是负责细菌中多种抗生素生物合成的大型多模块酶。这些大合酶的结构和机理分析可受益于发现以位点特异性方式识别单个结构域或接头的试剂。单克隆抗体不仅已经证明自己是类似应用中的主要工具,而且还具有以不可预测但通常有用的方式限制其靶标构象灵活性的额外好处。在这里,我们已经利用基于幼稚的人抗体库库发现A F AB(3A6)以高特异性识别6-脱氧赤藓醇内酯B合酶的末端硫酯酶(TE)结构域。生化分析用于验证3A6结合不会抑制酶转换。TE-3A6复合物的共晶体结构以2.45Å的分辨率确定,从而导致了这种蛋白质-蛋白质识别机制的原子表征。˚F AB绑定了对TE的结构完整性的影响最小。反过来,这些见解又被用于通过小角度X射线散射对与催化活性的PKS模块和双模块复合的3A6的溶液相构象进行研究。总之,我们开发了一种高亲和力的单克隆抗体工具,该工具可识别6-脱氧赤藓醇内酯B合酶的TE结构域,同时保持其天然功能。
更新日期:2018-10-05
中文翻译:

发现和表征的硫酯酶特异性单克隆抗体识别6-Deoxyerythronolide B合酶。
组装线聚酮化合物合酶(PKSs)是负责细菌中多种抗生素生物合成的大型多模块酶。这些大合酶的结构和机理分析可受益于发现以位点特异性方式识别单个结构域或接头的试剂。单克隆抗体不仅已经证明自己是类似应用中的主要工具,而且还具有以不可预测但通常有用的方式限制其靶标构象灵活性的额外好处。在这里,我们已经利用基于幼稚的人抗体库库发现A F AB(3A6)以高特异性识别6-脱氧赤藓醇内酯B合酶的末端硫酯酶(TE)结构域。生化分析用于验证3A6结合不会抑制酶转换。TE-3A6复合物的共晶体结构以2.45Å的分辨率确定,从而导致了这种蛋白质-蛋白质识别机制的原子表征。˚F AB绑定了对TE的结构完整性的影响最小。反过来,这些见解又被用于通过小角度X射线散射对与催化活性的PKS模块和双模块复合的3A6的溶液相构象进行研究。总之,我们开发了一种高亲和力的单克隆抗体工具,该工具可识别6-脱氧赤藓醇内酯B合酶的TE结构域,同时保持其天然功能。













































 京公网安备 11010802027423号
京公网安备 11010802027423号