当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration.
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-05 , DOI: 10.1038/s41467-018-06621-3 Amine Meliani 1, 2 , Florence Boisgerault 2 , Romain Hardet 1 , Solenne Marmier 1 , Fanny Collaud 2 , Giuseppe Ronzitti 2 , Christian Leborgne 2 , Helena Costa Verdera 1, 2 , Marcelo Simon Sola 1, 2 , Severine Charles 2 , Alban Vignaud 2 , Laetitia van Wittenberghe 2 , Giorgia Manni 3 , Olivier Christophe 4 , Francesca Fallarino 3 , Christopher Roy 5 , Alicia Michaud 5 , Petr Ilyinskii 5 , Takashi Kei Kishimoto 5 , Federico Mingozzi 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2018-10-05 , DOI: 10.1038/s41467-018-06621-3 Amine Meliani 1, 2 , Florence Boisgerault 2 , Romain Hardet 1 , Solenne Marmier 1 , Fanny Collaud 2 , Giuseppe Ronzitti 2 , Christian Leborgne 2 , Helena Costa Verdera 1, 2 , Marcelo Simon Sola 1, 2 , Severine Charles 2 , Alban Vignaud 2 , Laetitia van Wittenberghe 2 , Giorgia Manni 3 , Olivier Christophe 4 , Francesca Fallarino 3 , Christopher Roy 5 , Alicia Michaud 5 , Petr Ilyinskii 5 , Takashi Kei Kishimoto 5 , Federico Mingozzi 1, 2
Affiliation
|
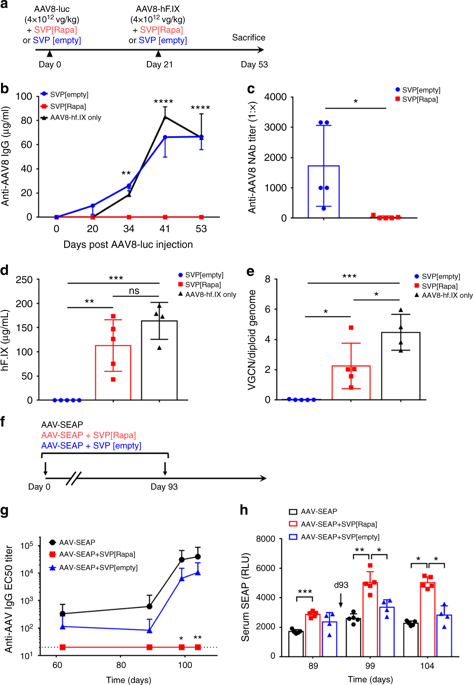
Gene therapy mediated by recombinant adeno-associated virus (AAV) vectors is a promising treatment for systemic monogenic diseases. However, vector immunogenicity represents a major limitation to gene transfer with AAV vectors, particularly for vector re-administration. Here, we demonstrate that synthetic vaccine particles encapsulating rapamycin (SVP[Rapa]), co-administered with AAV vectors, prevents the induction of anti-capsid humoral and cell-mediated responses. This allows successful vector re-administration in mice and nonhuman primates. SVP[Rapa] dosed with AAV vectors reduces B and T cell activation in an antigen-selective manner, inhibits CD8+ T cell infiltration in the liver, and efficiently blocks memory T cell responses. SVP[Rapa] immunomodulatory effects can be transferred from treated to naive mice by adoptive transfer of splenocytes, and is inhibited by depletion of CD25+ T cells, suggesting a role for regulatory T cells. Co-administration of SVP[Rapa] with AAV vector represents a powerful strategy to modulate vector immunogenicity and enable effective vector re-administration.
中文翻译:

使用致耐受性雷帕霉素纳米颗粒对 AAV 免疫原性进行抗原选择性调节能够成功地重新给药载体。
由重组腺相关病毒 (AAV) 载体介导的基因治疗是治疗系统性单基因疾病的一种有前途的治疗方法。然而,载体免疫原性是 AAV 载体基因转移的主要限制,特别是对于载体再给药。在这里,我们证明了包裹雷帕霉素 (SVP[Rapa]) 的合成疫苗颗粒与 AAV 载体共同给药,可防止诱导抗衣壳体液和细胞介导的反应。这允许在小鼠和非人类灵长类动物中成功地重新施用载体。与 AAV 载体一起给药的 SVP[Rapa] 以抗原选择性方式降低 B 和 T 细胞活化,抑制 CD8 +T 细胞浸润肝脏,并有效阻断记忆 T 细胞反应。SVP[Rapa] 免疫调节作用可以通过脾细胞的过继转移从处理过的小鼠转移到幼稚小鼠,并被 CD25 + T 细胞的消耗抑制,表明调节性 T 细胞的作用。SVP[Rapa] 与 AAV 载体的共同给药代表了调节载体免疫原性和实现有效载体再给药的强大策略。
更新日期:2018-10-06
中文翻译:

使用致耐受性雷帕霉素纳米颗粒对 AAV 免疫原性进行抗原选择性调节能够成功地重新给药载体。
由重组腺相关病毒 (AAV) 载体介导的基因治疗是治疗系统性单基因疾病的一种有前途的治疗方法。然而,载体免疫原性是 AAV 载体基因转移的主要限制,特别是对于载体再给药。在这里,我们证明了包裹雷帕霉素 (SVP[Rapa]) 的合成疫苗颗粒与 AAV 载体共同给药,可防止诱导抗衣壳体液和细胞介导的反应。这允许在小鼠和非人类灵长类动物中成功地重新施用载体。与 AAV 载体一起给药的 SVP[Rapa] 以抗原选择性方式降低 B 和 T 细胞活化,抑制 CD8 +T 细胞浸润肝脏,并有效阻断记忆 T 细胞反应。SVP[Rapa] 免疫调节作用可以通过脾细胞的过继转移从处理过的小鼠转移到幼稚小鼠,并被 CD25 + T 细胞的消耗抑制,表明调节性 T 细胞的作用。SVP[Rapa] 与 AAV 载体的共同给药代表了调节载体免疫原性和实现有效载体再给药的强大策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号