当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and enantiocontrol of axially chiral urazoles via organocatalytic tyrosine click reaction.
Nature Communications ( IF 14.7 ) Pub Date : 2016-Feb-11 , DOI: 10.1038/ncomms10677 Ji-Wei Zhang , Jin-Hui Xu , Dao-Juan Cheng , Chuan Shi , Xin-Yuan Liu , Bin Tan
Nature Communications ( IF 14.7 ) Pub Date : 2016-Feb-11 , DOI: 10.1038/ncomms10677 Ji-Wei Zhang , Jin-Hui Xu , Dao-Juan Cheng , Chuan Shi , Xin-Yuan Liu , Bin Tan
|
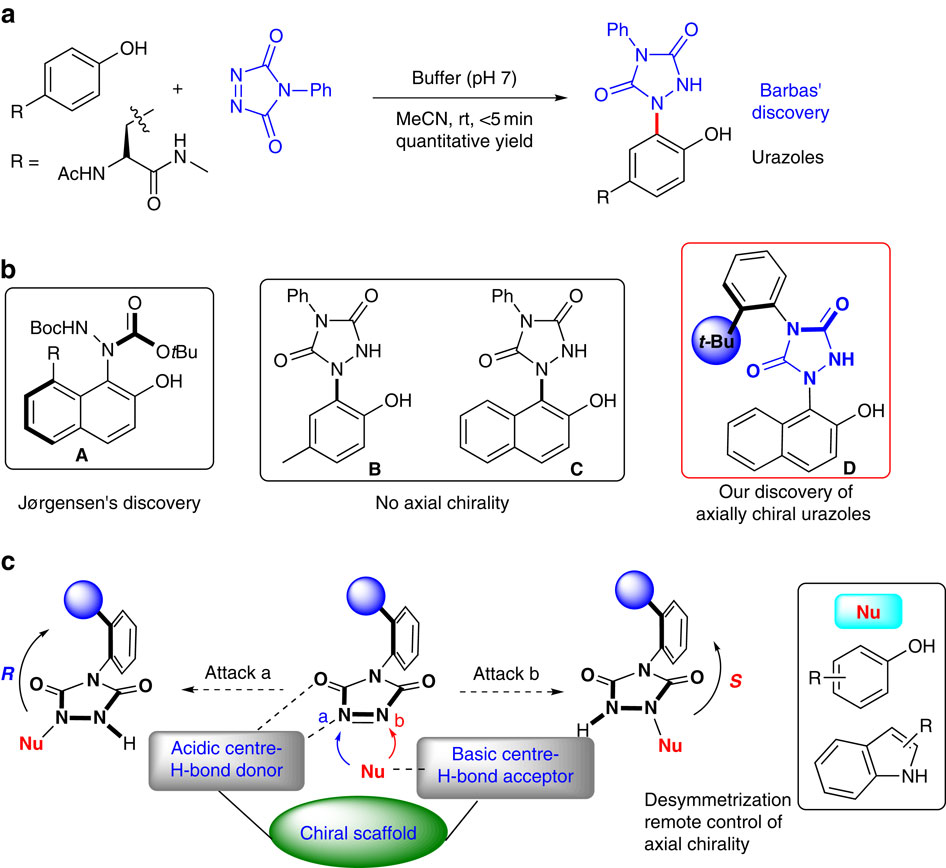
Axially chiral compounds play an important role in areas such as asymmetric catalysis. The tyrosine click-like reaction is an efficient approach for synthesis of urazoles with potential applications in pharmaceutical and asymmetric catalysis. Here we discover a class of urazole with axial chirality by restricted rotation around an N-Ar bond. By using bifunctional organocatalyst, we successfully develop an organocatalytic asymmetric tyrosine click-like reaction in high yields with excellent enantioselectivity under mild reaction conditions. The excellent remote enantiocontrol of the strategy originates from the efficient discrimination of the two reactive sites in the triazoledione and transferring the stereochemical information of the catalyst into the axial chirality of urazoles at the remote position far from the reactive site.
中文翻译:

通过有机催化酪氨酸点击反应发现和对映体轴向手性urazoles。
轴向手性化合物在不对称催化等领域中起着重要作用。酪氨酸的点击样反应是合成脲唑的有效方法,在药物和不对称催化中具有潜在的应用前景。在这里,我们发现了一类具有轴向手性的脲唑,通过围绕N-Ar键的受限旋转而形成。通过使用双功能有机催化剂,我们在温和的反应条件下成功地开发了高产率的有机催化不对称酪氨酸点击样反应,并具有出色的对映选择性。该策略出色的远程对映控制源于对三唑二酮中两个反应位点的有效区分,以及将催化剂的立体化学信息转移到远离反应位点的远端位置的尿嘧啶的轴向手性。
更新日期:2016-02-19
中文翻译:

通过有机催化酪氨酸点击反应发现和对映体轴向手性urazoles。
轴向手性化合物在不对称催化等领域中起着重要作用。酪氨酸的点击样反应是合成脲唑的有效方法,在药物和不对称催化中具有潜在的应用前景。在这里,我们发现了一类具有轴向手性的脲唑,通过围绕N-Ar键的受限旋转而形成。通过使用双功能有机催化剂,我们在温和的反应条件下成功地开发了高产率的有机催化不对称酪氨酸点击样反应,并具有出色的对映选择性。该策略出色的远程对映控制源于对三唑二酮中两个反应位点的有效区分,以及将催化剂的立体化学信息转移到远离反应位点的远端位置的尿嘧啶的轴向手性。































 京公网安备 11010802027423号
京公网安备 11010802027423号