当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential-Dependent Generation of O2– and LiO2 and Their Critical Roles in O2 Reduction to Li2O2 in Aprotic Li–O2 Batteries
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-12 00:00:00 , DOI: 10.1021/acs.jpcc.5b12338
Yelong Zhang 1, 2 , Xinmin Zhang 1 , Jiawei Wang 1 , William C. McKee 3 , Ye Xu 3 , Zhangquan Peng 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-12 00:00:00 , DOI: 10.1021/acs.jpcc.5b12338
Yelong Zhang 1, 2 , Xinmin Zhang 1 , Jiawei Wang 1 , William C. McKee 3 , Ye Xu 3 , Zhangquan Peng 1
Affiliation
|
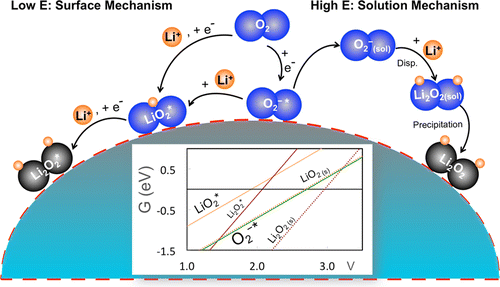
Discharging of the aprotic Li–O2 battery relies on the oxygen reduction reaction (ORR) producing Li2O2 in the positive electrode, which remains incompletely understood. Here, we report a mechanistic study of the Li-ORR on a model system, i.e., an Au electrode in a Li+ dimethyl sulfoxide (DMSO) electrolyte. By spectroscopic identification of the reaction intermediates coupled with density functional theory calculations, we conclude that the formation of O2– and LiO2 in the Li-ORR critically depends on electrode potentials and determines the Li2O2 formation mechanism. At low overpotentials (> 2.0 V vs Li/Li+) O2– is identified to be the first surface intermediate, which diffuses into the bulk electrolyte and forms Li2O2 therein via a solution-mediated disproportionation mechanism. At high overpotentials (ca. 2.0–1.6 V vs Li/Li+) LiO2 has been observed, which can rapidly transform to Li2O2 by further electro-reduction, suggesting a surface-mediated mechanism. The solution-mediated Li2O2 formation that can account for the widely observed toroid-shaped discharged Li2O2 particles has also been thoroughly examined. Thus, O2– formation controls the overall reaction onset potential, and LiO2 formation demarcates the change from a solution- to surface-mediated reaction mechanism. The new findings and improved understandings of the Li-ORR in DMSO will contribute to the further development of aprotic Li–O2 batteries.
中文翻译:

的O-潜在的依赖性产生2 -和LIO 2及其Ø关键作用2减少到李2 Ø 2在非质子LIO 2电池
非质子传递的Li–O 2电池的放电依赖于氧还原反应(ORR)在正极中产生Li 2 O 2,这一点尚不完全清楚。在这里,我们报告了在模型系统(即Li +二甲基亚砜(DMSO)电解质中的Au电极)上进行Li-ORR的机理研究。通过加上密度泛函理论计算的反应中间体的光谱鉴定,我们得出结论的O的形成2 -和LIO 2中的Li ORR关键取决于电极电位,并且确定李2 ö 2形成机制。在低电势下(> 2.0 V vs Li / Li +)O 2 –被确定为第一个表面中间体,它通过溶液介导的歧化机理扩散到本体电解质中并在其中形成Li 2 O 2。在高电势下(相对于Li / Li +约为2.0–1.6 V ),观察到LiO 2可以通过进一步的电还原迅速转变为Li 2 O 2,这表明是表面介导的机制。还已经彻底研究了溶液介导的Li 2 O 2的形成,该形成可以解释广泛观察到的环形放电的Li 2 O 2颗粒。因此,O 2 –LiO 2的形成控制着整个反应的开始电位,而LiO 2的形成则界定了从溶液到表面介导的反应机理的变化。在DMSO中对Li-ORR的新发现和改进的理解将有助于进一步发展非质子传递Li-O 2电池。
更新日期:2016-02-12
中文翻译:

的O-潜在的依赖性产生2 -和LIO 2及其Ø关键作用2减少到李2 Ø 2在非质子LIO 2电池
非质子传递的Li–O 2电池的放电依赖于氧还原反应(ORR)在正极中产生Li 2 O 2,这一点尚不完全清楚。在这里,我们报告了在模型系统(即Li +二甲基亚砜(DMSO)电解质中的Au电极)上进行Li-ORR的机理研究。通过加上密度泛函理论计算的反应中间体的光谱鉴定,我们得出结论的O的形成2 -和LIO 2中的Li ORR关键取决于电极电位,并且确定李2 ö 2形成机制。在低电势下(> 2.0 V vs Li / Li +)O 2 –被确定为第一个表面中间体,它通过溶液介导的歧化机理扩散到本体电解质中并在其中形成Li 2 O 2。在高电势下(相对于Li / Li +约为2.0–1.6 V ),观察到LiO 2可以通过进一步的电还原迅速转变为Li 2 O 2,这表明是表面介导的机制。还已经彻底研究了溶液介导的Li 2 O 2的形成,该形成可以解释广泛观察到的环形放电的Li 2 O 2颗粒。因此,O 2 –LiO 2的形成控制着整个反应的开始电位,而LiO 2的形成则界定了从溶液到表面介导的反应机理的变化。在DMSO中对Li-ORR的新发现和改进的理解将有助于进一步发展非质子传递Li-O 2电池。

































 京公网安备 11010802027423号
京公网安备 11010802027423号