当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of hierarchically-ordered nanoporous silver foam and its electrocatalytic properties in reductive dehalogenation of organic compounds†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-09-22 00:00:00 , DOI: 10.1039/c8nj03460e Andrey M. Mishura 1, 2, 3 , Anton S. Lytvynenko 1, 2, 3 , Konstantin S. Gavrilenko 2, 3, 4, 5, 6 , Alexander E. Baranchikov 7, 8, 9 , Natalia V. Grabovaya 1, 2, 3 , Mikhail A. Kiskin 7, 8, 9 , Sergey V. Kolotilov 1, 2, 3
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-09-22 00:00:00 , DOI: 10.1039/c8nj03460e Andrey M. Mishura 1, 2, 3 , Anton S. Lytvynenko 1, 2, 3 , Konstantin S. Gavrilenko 2, 3, 4, 5, 6 , Alexander E. Baranchikov 7, 8, 9 , Natalia V. Grabovaya 1, 2, 3 , Mikhail A. Kiskin 7, 8, 9 , Sergey V. Kolotilov 1, 2, 3
Affiliation
|
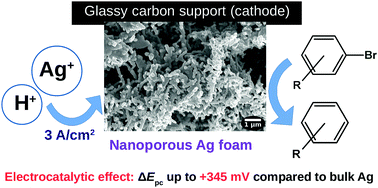
Nanoporous silver foam on a glassy carbon electrode (AgNF/GC) was formed by cathodic deposition of silver from an acidic solution of AgBF4 at high current density. The material can be described (based on results of optical microscopy, SEM, TEM and XRD investigation) as hierarchically (micro/nano) porous matter with ca. 40 nm silver crystals assembled in irregular ca. 70–300 nm thick filaments arranged in a foam-like structure with ca. 20 μm cavities and ca. 7 μm walls. The AgNF surface area was estimated by pseudocapacitance measurement as ca. 12 times higher than the geometrical area. Reduction of bromobenzene and other aryl bromides containing redox-inactive (F-, CH3-, CH3O-) or redox-active (–NO2, –CN and CH3CO-) substituents, as well as alkyl bromides (CF3CHClBr, CF2Br2) on AgNF/GC was studied. The peak potentials of the processes assigned to debromination of the organic halides were less negative (up to +345 mV) than on smooth silver, indicating superior electrocatalytic properties of the nanoporous silver foam. Comparison of the CV peak currents of the processes on AgNF/GC and smooth silver, as well as their changes in cycling in different regimes allowed formation of a few kinds of electrocatalytically active sites on the AgNF surface to be suggested. Electrolysis of 1-bromo-4-fluorobenzene on the AgNF electrode led to fluorobenzene, and less than 1 mg of AgNF revealed performance comparable to 500 mg of smooth silver wire. The results can be utilized for creation of electrochemical sensors as well as for preparative detoxification of halogen-containing persistent organic pollutants.
中文翻译:

分层纳米多孔银泡沫的形成及其在有机化合物还原脱卤中的电催化性能†
通过在高电流密度下从AgBF 4的酸性溶液中阴极银的沉积,在玻璃碳电极(AgNF / GC)上形成纳米多孔银泡沫。可以将这种材料描述为(基于光学显微镜,SEM,TEM和XRD研究的结果)为分层的(微/纳米)多孔物质。40 nm的银晶体以不规则的方式组装而成。70-300 nm厚的长丝以泡沫状结构排列,约有 20μm的腔和大约 壁厚7μm。通过假电容测量将AgNF表面积估计为约。比几何面积高12倍。还原无氧化还原活性的溴苯和其他芳基溴化物(F-,CH 3-,CH 3 O-)或氧化还原活性(-NO 2,-CN和CH 3 CO-)取代基,以及烷基溴(CF 3 CHClBr,CF 2 Br 2)在AgNF / GC上进行了研究。与光滑的银相比,分配给有机卤化物脱溴的方法的峰值电势负性较小(最高+345 mV),表明纳米多孔银泡沫具有优异的电催化性能。比较AgNF / GC和平滑银上的过程的CV峰值电流,以及它们在不同状态下的循环变化,可以建议在AgNF表面上形成几种电催化活性位点。在AgNF电极上电解1-溴-4-氟苯会生成氟苯,而少于1 mg的AgNF则显示出与500 mg光滑的银线相当的性能。该结果可用于创建电化学传感器以及用于含卤素的持久性有机污染物的制备性解毒。
更新日期:2018-09-22
中文翻译:

分层纳米多孔银泡沫的形成及其在有机化合物还原脱卤中的电催化性能†
通过在高电流密度下从AgBF 4的酸性溶液中阴极银的沉积,在玻璃碳电极(AgNF / GC)上形成纳米多孔银泡沫。可以将这种材料描述为(基于光学显微镜,SEM,TEM和XRD研究的结果)为分层的(微/纳米)多孔物质。40 nm的银晶体以不规则的方式组装而成。70-300 nm厚的长丝以泡沫状结构排列,约有 20μm的腔和大约 壁厚7μm。通过假电容测量将AgNF表面积估计为约。比几何面积高12倍。还原无氧化还原活性的溴苯和其他芳基溴化物(F-,CH 3-,CH 3 O-)或氧化还原活性(-NO 2,-CN和CH 3 CO-)取代基,以及烷基溴(CF 3 CHClBr,CF 2 Br 2)在AgNF / GC上进行了研究。与光滑的银相比,分配给有机卤化物脱溴的方法的峰值电势负性较小(最高+345 mV),表明纳米多孔银泡沫具有优异的电催化性能。比较AgNF / GC和平滑银上的过程的CV峰值电流,以及它们在不同状态下的循环变化,可以建议在AgNF表面上形成几种电催化活性位点。在AgNF电极上电解1-溴-4-氟苯会生成氟苯,而少于1 mg的AgNF则显示出与500 mg光滑的银线相当的性能。该结果可用于创建电化学传感器以及用于含卤素的持久性有机污染物的制备性解毒。































 京公网安备 11010802027423号
京公网安备 11010802027423号