当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flexible Peptide Linkers Enhance the Antimicrobial Activity of Surface-Immobilized Bacteriolytic Enzymes
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acsami.8b14411 Xia Wu , Keith Fraser , Jian Zha , Jonathan S. Dordick
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acsami.8b14411 Xia Wu , Keith Fraser , Jian Zha , Jonathan S. Dordick
|
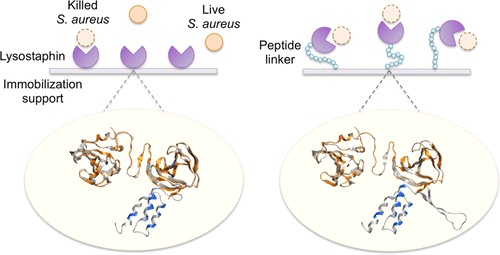
Chemical linkers are frequently used in enzyme immobilization to improve enzyme flexibility and activity, whereas peptide linkers, although ubiquitous in protein engineering, are much less explored in enzyme immobilization. Here, we report peptide-linker-assisted noncovalent immobilization of the bacteriolytic enzyme lysostaphin (Lst) to generate anti-Staphylococcus aureus surfaces. Lst was immobilized through affinity tags onto a silica surface (glass slides) and nickel nitrilotriacetic acid (NiNTA) agarose beads via silica-binding peptides (SiBPs) or a hexahistidine tag (His-tag) fused at the C-terminus of Lst, respectively. By inserting specific peptide linkers upstream of the SiBP or His-tag, the immobilized enzymes killed >99.5% of S. aureus ATCC 6538 cells (108 CFU/mL) within 3 h in buffer and could be reused multiple times without significant loss of activity. In contrast, immobilized Lst without a peptide linker was less active/stable. Molecular modeling of Lst–linker–affinity tag constructs illustrated that the presence of the peptide linkers enhanced the molecular flexibility of the proximal Lst binding domain, which interacts with the bacterial substrate, and such increased flexibility correlated with increased antimicrobial activity. We further show that Lst immobilized onto NiNTA beads retained the ability to kill ∼99% of a 108 CFU/mL microbial challenge even in the presence of 1% of a commercial anionic surfactant, C12-14 alcohol EO 3:1 sodium sulfate, when the Lst construct contained a decapeptide linker containing glycine, serine, and alanine residues. This linker-assisted immobilization strategy could be extended to an unrelated lytic enzyme, the endolysin PlyPH, to target Bacillus anthracis Sterne cells either in buffer or in the presence of anionic surfactants. Our approach, therefore, provides a facile route to the use of antimicrobial enzymes on surfaces.
中文翻译:

灵活的肽接头增强表面固定的细菌分解酶的抗菌活性
化学接头经常用于酶固定化,以提高酶的柔韧性和活性,而肽接头虽然在蛋白质工程中普遍存在,但在酶固定化方面却少有研究。在这里,我们报告溶菌酶溶葡萄球菌素(Lst)的肽接头辅助非共价固定化,以产生抗金黄色葡萄球菌表面。通过亲和标签将Lst分别通过在Lst C端融合的二氧化硅结合肽(SiBPs)或六组氨酸标签(His-tag),通过亲和标签固定在二氧化硅表面(载玻片)和次氮基三乙酸镍(NiNTA)琼脂糖珠上。 。通过在SiBP或His-tag的上游插入特定的肽接头,固定的酶杀死了> 99.5%的金黄色葡萄球菌ATCC 6538细胞(10 8CFU / mL)在缓冲液中3小时之内,可以重复使用多次,而不会显着降低活性。相反,没有肽接头的固定化Lst的活性/稳定性较差。Lst-连接子-亲和标签构建体的分子建模表明,肽连接子的存在增强了与细菌底物相互作用的近端Lst结合域的分子柔性,并且这种增加的柔性与抗菌活性的提高相关。我们进一步表明,固定在NiNTA磁珠上的Lst保留了杀死10 8个细胞中约99%的能力。当Lst构建体包含含有甘氨酸,丝氨酸和丙氨酸残基的十肽接头时,即使在1%的工业阴离子表面活性剂C12-14醇EO 3:1硫酸钠的存在下,CFU / mL微生物的攻击也是如此。这种接头辅助的固定策略可以扩展到无关的裂解酶,即内溶素PlyPH,在缓冲液中或在存在阴离子表面活性剂的情况下靶向炭疽芽孢杆菌Sterne细胞。因此,我们的方法为在表面上使用抗微生物酶提供了一条简便的途径。
更新日期:2018-10-03
中文翻译:

灵活的肽接头增强表面固定的细菌分解酶的抗菌活性
化学接头经常用于酶固定化,以提高酶的柔韧性和活性,而肽接头虽然在蛋白质工程中普遍存在,但在酶固定化方面却少有研究。在这里,我们报告溶菌酶溶葡萄球菌素(Lst)的肽接头辅助非共价固定化,以产生抗金黄色葡萄球菌表面。通过亲和标签将Lst分别通过在Lst C端融合的二氧化硅结合肽(SiBPs)或六组氨酸标签(His-tag),通过亲和标签固定在二氧化硅表面(载玻片)和次氮基三乙酸镍(NiNTA)琼脂糖珠上。 。通过在SiBP或His-tag的上游插入特定的肽接头,固定的酶杀死了> 99.5%的金黄色葡萄球菌ATCC 6538细胞(10 8CFU / mL)在缓冲液中3小时之内,可以重复使用多次,而不会显着降低活性。相反,没有肽接头的固定化Lst的活性/稳定性较差。Lst-连接子-亲和标签构建体的分子建模表明,肽连接子的存在增强了与细菌底物相互作用的近端Lst结合域的分子柔性,并且这种增加的柔性与抗菌活性的提高相关。我们进一步表明,固定在NiNTA磁珠上的Lst保留了杀死10 8个细胞中约99%的能力。当Lst构建体包含含有甘氨酸,丝氨酸和丙氨酸残基的十肽接头时,即使在1%的工业阴离子表面活性剂C12-14醇EO 3:1硫酸钠的存在下,CFU / mL微生物的攻击也是如此。这种接头辅助的固定策略可以扩展到无关的裂解酶,即内溶素PlyPH,在缓冲液中或在存在阴离子表面活性剂的情况下靶向炭疽芽孢杆菌Sterne细胞。因此,我们的方法为在表面上使用抗微生物酶提供了一条简便的途径。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号