当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sacrificial Cobalt-carbon Bond Homolysis in Coenzyme B12 as a Cofactor Conservation Strategy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-03 , DOI: 10.1021/jacs.8b08659 Gregory C Campanello 1 , Markus Ruetz 1 , Greg J Dodge 1, 2 , Harsha Gouda 1, 3 , Aditi Gupta 1 , Umar T Twahir 4 , Michelle M Killian 5 , David Watkins 6 , David S Rosenblatt 6 , Thomas C Brunold 5 , Kurt Warncke 4 , Janet L Smith 1, 2 , Ruma Banerjee 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-03 , DOI: 10.1021/jacs.8b08659 Gregory C Campanello 1 , Markus Ruetz 1 , Greg J Dodge 1, 2 , Harsha Gouda 1, 3 , Aditi Gupta 1 , Umar T Twahir 4 , Michelle M Killian 5 , David Watkins 6 , David S Rosenblatt 6 , Thomas C Brunold 5 , Kurt Warncke 4 , Janet L Smith 1, 2 , Ruma Banerjee 1
Affiliation
|
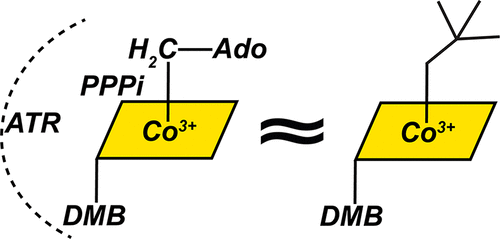
A sophisticated intracellular trafficking pathway in humans is used to tailor vitamin B12 into its active cofactor forms, and to deliver it to two known B12-dependent enzymes. Herein, we report an unexpected strategy for cellular retention of B12, an essential and reactive cofactor. If methylmalonyl-CoA mutase is unavailable to accept the coenzyme B12 product of adenosyltransferase, the latter catalyzes homolytic scission of the cobalt-carbon bond in an unconventional reversal of the nucleophilic displacement reaction that was used to make it. The resulting homolysis product binds more tightly to adenosyltransferase than does coenzyme B12, facilitating cofactor retention. We have trapped, and characterized spectroscopically, an intermediate in which the cobalt-carbon bond is weakened prior to being broken. The physiological relevance of this sacrificial catalytic activity for cofactor retention is supported by the significantly lower coenzyme B12 concentration in patients with dysfunctional methylmalonyl-CoA mutase but normal adenosyltransferase activity.
中文翻译:

辅酶 B12 中的牺牲钴-碳键均裂作为辅因子保护策略
人类复杂的细胞内运输途径用于将维生素 B12 调整为其活性辅因子形式,并将其传递给两种已知的 B12 依赖性酶。在这里,我们报告了一种意想不到的 B12 细胞保留策略,B12 是一种必不可少的反应性辅因子。如果甲基丙二酰辅酶A变位酶不能接受腺苷转移酶的辅酶B12产物,后者催化钴-碳键的均裂断裂,这是用于制造它的亲核置换反应的非常规逆转。与辅酶 B12 相比,所得的均解产物与腺苷转移酶的结合更紧密,从而促进辅因子的保留。我们已经捕获并用光谱表征了一种中间体,其中钴 - 碳键在断裂之前被削弱。
更新日期:2018-10-03
中文翻译:

辅酶 B12 中的牺牲钴-碳键均裂作为辅因子保护策略
人类复杂的细胞内运输途径用于将维生素 B12 调整为其活性辅因子形式,并将其传递给两种已知的 B12 依赖性酶。在这里,我们报告了一种意想不到的 B12 细胞保留策略,B12 是一种必不可少的反应性辅因子。如果甲基丙二酰辅酶A变位酶不能接受腺苷转移酶的辅酶B12产物,后者催化钴-碳键的均裂断裂,这是用于制造它的亲核置换反应的非常规逆转。与辅酶 B12 相比,所得的均解产物与腺苷转移酶的结合更紧密,从而促进辅因子的保留。我们已经捕获并用光谱表征了一种中间体,其中钴 - 碳键在断裂之前被削弱。































 京公网安备 11010802027423号
京公网安备 11010802027423号