当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption Mechanism of Molybdenum(VI) on Manganese Oxides Causing a Large Isotope Fractionation
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00090 Masato Tanaka 1 , Daisuke Ariga 2 , Teruhiko Kashiwabara 3 , Yoshio Takahashi 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00090 Masato Tanaka 1 , Daisuke Ariga 2 , Teruhiko Kashiwabara 3 , Yoshio Takahashi 1
Affiliation
|
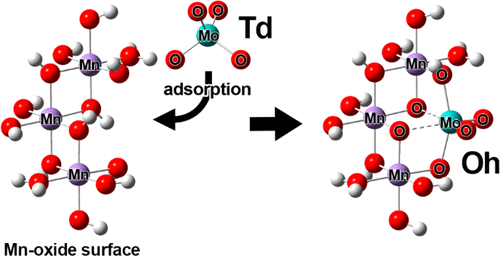
A large mass-dependent isotope fractionation of molybdenum (Mo) during its adsorption on marine ferromanganese oxides, which are mixtures of iron (Fe) (oxyhydr)oxide and manganese (Mn) oxide, is most likely caused by a geometrical change from tetrahedral (Td) to distorted octahedral (Oh) during adsorption based on X-ray absorption fine structure (XAFS) spectroscopy. However, the understanding of such fractionation can be enhanced further. In this study, density functional theory (DFT) calculations were conducted to examine models of distorted Oh species on Mn oxide which are related to our proposed adsorption mechanism of molybdate. Results showed that even monomeric adsorbed species can reasonably explain highly distorted geometries observed by XAFS spectroscopy with large isotope fractionation. This finding indicates that the formation of polymolybdate, such as heptamolybdate, under circumneutral experimental condition (pH ∼ 8) at low Mo concentration in seawater is not required to explain the experimental results. Two elemental properties of Mo(VI), namely d0 electron configuration and appropriate ionic radius, led to the formation of its distorted Oh adsorbed species based on the observation of highly distorted adsorbed species with DFT calculation results. This finding indicates that molybdate adsorption on Mn oxide occurs via addition reaction, which can explain the following natural phenomena: (i) Mn oxide is the host phase of molybdate in marine ferromanganese oxides, although most negatively charged ions are preferentially adsorbed on Fe (oxyhydr)oxides. (ii) The large isotope fractionation of Mo is observed during its adsorption on natural ferromanganese oxides through the formation of distorted Oh adsorbed species on Mn oxide.
中文翻译:

大型同位素分馏过程中钼(VI)在锰氧化物上的吸附机理
钼(Mo)吸附在海洋氧化铁锰上的质量依存的同位素分级较大,这是氧化铁(Fe)(OH)和氧化锰(Mn)的混合物,最有可能是由于四面体( Td)基于X射线吸收精细结构(XAFS)光谱在吸附过程中变形为八面体(Oh)。但是,可以进一步增强对这种分馏的理解。在这项研究中,进行了密度泛函理论(DFT)计算,以检验与我们提出的钼酸盐吸附机理有关的在Mn氧化物上扭曲的Oh物种的模型。结果表明,即使单体吸附的物质也可以合理地解释XAFS光谱观察到的具有较大同位素分馏的高度畸变的几何形状。这一发现表明,在海水中低Mo浓度下,在环境中性实验条件(pH约为8)下,聚钼酸盐(如七钼酸盐)的形成并不需要解释实验结果。Mo(VI)的两个元素性质,即d0电子构型和适当的离子半径,基于用DFT计算结果观察到的高度畸变的吸附物种,导致其畸变的Oh吸附物种的形成。这一发现表明,钼酸通过加成反应吸附在Mn氧化物上,这可以解释以下自然现象:(i)Mn氧化物是船用锰铁氧化物中钼酸盐的主体相,尽管大多数带负电的离子优先吸附在Fe(羟基氧化物)上。 )氧化物。(ii)在钼吸附在天然锰铁氧化物上时,通过在锰氧化物上形成扭曲的Oh吸附物质,观察到了较大的同位素分馏。
更新日期:2018-10-02
中文翻译:

大型同位素分馏过程中钼(VI)在锰氧化物上的吸附机理
钼(Mo)吸附在海洋氧化铁锰上的质量依存的同位素分级较大,这是氧化铁(Fe)(OH)和氧化锰(Mn)的混合物,最有可能是由于四面体( Td)基于X射线吸收精细结构(XAFS)光谱在吸附过程中变形为八面体(Oh)。但是,可以进一步增强对这种分馏的理解。在这项研究中,进行了密度泛函理论(DFT)计算,以检验与我们提出的钼酸盐吸附机理有关的在Mn氧化物上扭曲的Oh物种的模型。结果表明,即使单体吸附的物质也可以合理地解释XAFS光谱观察到的具有较大同位素分馏的高度畸变的几何形状。这一发现表明,在海水中低Mo浓度下,在环境中性实验条件(pH约为8)下,聚钼酸盐(如七钼酸盐)的形成并不需要解释实验结果。Mo(VI)的两个元素性质,即d0电子构型和适当的离子半径,基于用DFT计算结果观察到的高度畸变的吸附物种,导致其畸变的Oh吸附物种的形成。这一发现表明,钼酸通过加成反应吸附在Mn氧化物上,这可以解释以下自然现象:(i)Mn氧化物是船用锰铁氧化物中钼酸盐的主体相,尽管大多数带负电的离子优先吸附在Fe(羟基氧化物)上。 )氧化物。(ii)在钼吸附在天然锰铁氧化物上时,通过在锰氧化物上形成扭曲的Oh吸附物质,观察到了较大的同位素分馏。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号