当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Continuous Spherical Crystallization to Prepare Fenofibrate Agglomerates with Impurity Complexation Using Mixed-Suspension, Mixed-Product Removal Crystallizer
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acs.cgd.8b00426 Kohei Tahara 1 , Yuichi Kono 1 , Allan S. Myerson 2 , Hirofumi Takeuchi 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acs.cgd.8b00426 Kohei Tahara 1 , Yuichi Kono 1 , Allan S. Myerson 2 , Hirofumi Takeuchi 1
Affiliation
|
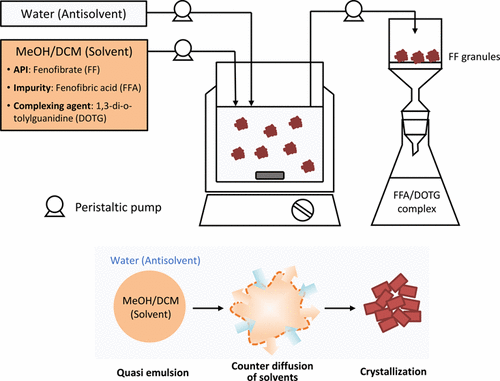
Agglomeration during crystallization, such as spherical crystallization, has the potential to integrate pharmaceutical downstream processes after compound synthesis. This process incorporates granulation and agglomeration during the crystallization process and to date has been performed in batch reactors. However, because it involves certain complicated phenomena, the batch process is difficult to scale up for commercial production. We demonstrate the feasibility of a continuous process of crystallization with agglomeration based on the spherical crystallization of fenofibrate (FF) as a model active pharmaceutical ingredient (API) by using a mixed-suspension, mixed-product removal (MSMPR) crystallizer. The characteristics of FF granules produced by the emulsion solvent diffusion method with MSMPR were compared with the characteristics of batch-prepared FF granules. The particle-size distribution of the FF agglomerates prepared by MSMPR crystallizer was not significantly different from that prepared by the equivalent batch-wise crystallizer. The effects of the operating parameters in MSMPR were examined based on the crystal properties of FF granules produced via continuous spherical crystallization. Conversely, pharmaceutical crystallization mainly is purification to generate pure solid APIs. However, impurity separation in spherical crystallization has not been demonstrated. Impurity inclusion in the crystal lattice is difficult to avoid, especially when the impurity and targeted API molecule structures are similar. Thus, we established effective purification during continuous spherical crystallization using a complexing agent (1,3-di-o-tolylguanidine, DOTG) with a representative impurity (fenofibric acid, FFA) to prevent impurity incorporation into the crystal lattice. Purification improvement using the FFA/DOTG complex was substantiated via continuous spherical crystallization with MSMPR.
中文翻译:

使用混合悬浮,混合产物去除结晶器开发连续球形结晶以制备具有杂质络合的非诺贝特凝聚物
结晶过程中的团聚(例如球形结晶)具有在化合物合成后整合药物下游工艺的潜力。该方法在结晶过程中结合了制粒和附聚作用,并且迄今为止已经在间歇式反应器中进行。但是,由于涉及某些复杂的现象,因此分批处理难以大规模推广用于商业生产。我们通过使用混合悬浮,混合产品去除(MSMPR)结晶器,证明了以非诺贝特(FF)作为模型活性药物成分(API)的球形结晶为基础的,连续附聚结晶过程的可行性。将乳液溶剂扩散法与MSMPR制备的FF颗粒的特性与分批制备的FF颗粒的特性进行了比较。通过MSMPR结晶器制备的FF附聚物的粒度分布与通过等效分批结晶器制备的FF附聚物的粒度分布没有显着差异。基于通过连续球形结晶生产的FF颗粒的晶体特性,检查了MSMPR中操作参数的影响。相反,药物结晶主要是纯化以生成纯固体API。然而,尚未证明球形结晶中的杂质分离。很难避免杂质包含在晶格中,尤其是当杂质和目标API分子结构相似时。因此,邻甲苯基胍,DOTG)具有代表性的杂质(非诺贝特酸,FFA),以防止杂质掺入晶格中。通过使用MSMPR进行连续球状结晶,证实了使用FFA / DOTG复合物的纯化效果得到了改善。
更新日期:2018-10-01
中文翻译:

使用混合悬浮,混合产物去除结晶器开发连续球形结晶以制备具有杂质络合的非诺贝特凝聚物
结晶过程中的团聚(例如球形结晶)具有在化合物合成后整合药物下游工艺的潜力。该方法在结晶过程中结合了制粒和附聚作用,并且迄今为止已经在间歇式反应器中进行。但是,由于涉及某些复杂的现象,因此分批处理难以大规模推广用于商业生产。我们通过使用混合悬浮,混合产品去除(MSMPR)结晶器,证明了以非诺贝特(FF)作为模型活性药物成分(API)的球形结晶为基础的,连续附聚结晶过程的可行性。将乳液溶剂扩散法与MSMPR制备的FF颗粒的特性与分批制备的FF颗粒的特性进行了比较。通过MSMPR结晶器制备的FF附聚物的粒度分布与通过等效分批结晶器制备的FF附聚物的粒度分布没有显着差异。基于通过连续球形结晶生产的FF颗粒的晶体特性,检查了MSMPR中操作参数的影响。相反,药物结晶主要是纯化以生成纯固体API。然而,尚未证明球形结晶中的杂质分离。很难避免杂质包含在晶格中,尤其是当杂质和目标API分子结构相似时。因此,邻甲苯基胍,DOTG)具有代表性的杂质(非诺贝特酸,FFA),以防止杂质掺入晶格中。通过使用MSMPR进行连续球状结晶,证实了使用FFA / DOTG复合物的纯化效果得到了改善。






























 京公网安备 11010802027423号
京公网安备 11010802027423号