当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Study of Lithium Titanate as a Possible Cathode Material for Solid-State Lithium–Sulfur Batteries
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-04-28 00:00:00 , DOI: 10.1021/jp5105455 Valéry Weber 1 , Teodoro Laino 1 , Alessandro Curioni 1 , Thomas Eckl 2 , Christine Engel 2 , Jitti Kasemchainan 2 , Nils Salingue 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-04-28 00:00:00 , DOI: 10.1021/jp5105455 Valéry Weber 1 , Teodoro Laino 1 , Alessandro Curioni 1 , Thomas Eckl 2 , Christine Engel 2 , Jitti Kasemchainan 2 , Nils Salingue 2
Affiliation
|
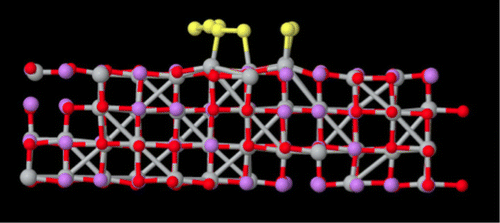
LiS cells are currently built with metallic lithium as anode, a liquid electrolyte, and a cathode composed of a mixture of sulfur, carbon, and binder. While this type of cell produces good capacity during the early cycles, unwanted reactions with the electrolyte degrade the cathode and anode, making the whole cell not competitive with Li-ion batteries. A viable solution to mitigate this problem is the replacement of the carbon, binder, and electrolyte with a ceramic matrix, with high electronic and ionic conductivity. Lithium titanate (Li4Ti5O12) spinel may be a potential candidate for the fabrication of composite cathodes, due to its mechanical robustness and its high electronic and Li-ion conductivity. In this paper, we present an ab initio molecular dynamics study complemented with experimental investigations, offering a novel interpretation for the Li-ion mobility in Li4Ti5O12 and Li7Ti5O12 as well as for the chemical reactivity of these materials with molecular sulfur. Also, we present a model for the passivation of Li4Ti5O12 and Li7Ti5O12 surfaces by lithium carbonate, addressing both Li-ion mobility at the interface and sulfur reactivity. On the basis of our results, the deployment of Li4Ti5O12 and Li7Ti5O12 materials for sulfur-based battery technology is questioned mainly by the lower Li-ion conductivity of the carbonate-passivated surfaces and by the chemical reactivity of Li7Ti5O12 with sulfur molecules, which would lead to self-discharge, with resulting loss of capacity and inferior battery performance.
中文翻译:

钛酸锂作为固态锂硫电池正极材料的计算研究
目前,LiS电池是用金属锂作为阳极,液体电解质和由硫,碳和粘合剂的混合物组成的阴极构建的。尽管此类电池在早期循环中会产生良好的容量,但与电解液发生的不良反应会使阴极和阳极退化,从而使整个电池无法与锂离子电池竞争。缓解此问题的可行解决方案是用具有高电子和离子导电性的陶瓷基质代替碳,粘合剂和电解质。钛酸锂(Li 4 Ti 5 O 12)尖晶石由于其机械强度,高电子和锂离子传导性而可能成为制造复合阴极的潜在候选者。在本文中,我们介绍一个从头开始分子动力学研究与实验研究相辅相成,为Li 4 Ti 5 O 12和Li 7 Ti 5 O 12中的锂离子迁移以及这些材料与分子硫的化学反应性提供了新颖的解释。此外,我们提出了一种通过碳酸锂对Li 4 Ti 5 O 12和Li 7 Ti 5 O 12表面进行钝化的模型,解决了界面处的锂离子迁移率和硫反应性的问题。根据我们的结果,部署Li 4 Ti 5 O 12硫基电池技术使用的Li 7 Ti 5 O 12和材料受到质疑的主要原因是碳酸盐钝化表面的锂离子电导率较低,以及Li 7 Ti 5 O 12与硫分子的化学反应性,这将导致自放电,从而导致容量损失和劣质的电池性能。
更新日期:2015-04-28
中文翻译:

钛酸锂作为固态锂硫电池正极材料的计算研究
目前,LiS电池是用金属锂作为阳极,液体电解质和由硫,碳和粘合剂的混合物组成的阴极构建的。尽管此类电池在早期循环中会产生良好的容量,但与电解液发生的不良反应会使阴极和阳极退化,从而使整个电池无法与锂离子电池竞争。缓解此问题的可行解决方案是用具有高电子和离子导电性的陶瓷基质代替碳,粘合剂和电解质。钛酸锂(Li 4 Ti 5 O 12)尖晶石由于其机械强度,高电子和锂离子传导性而可能成为制造复合阴极的潜在候选者。在本文中,我们介绍一个从头开始分子动力学研究与实验研究相辅相成,为Li 4 Ti 5 O 12和Li 7 Ti 5 O 12中的锂离子迁移以及这些材料与分子硫的化学反应性提供了新颖的解释。此外,我们提出了一种通过碳酸锂对Li 4 Ti 5 O 12和Li 7 Ti 5 O 12表面进行钝化的模型,解决了界面处的锂离子迁移率和硫反应性的问题。根据我们的结果,部署Li 4 Ti 5 O 12硫基电池技术使用的Li 7 Ti 5 O 12和材料受到质疑的主要原因是碳酸盐钝化表面的锂离子电导率较低,以及Li 7 Ti 5 O 12与硫分子的化学反应性,这将导致自放电,从而导致容量损失和劣质的电池性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号