当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amorphous CaCO3: Influence of the formation time on its degree of hydration and stability
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b08298 Huachuan Du 1 , Mathias Steinacher 1 , Camelia Borca 2 , Thomas Huthwelker 2 , Anna Murello 3 , Francesco Stellacci 3 , Esther Amstad 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b08298 Huachuan Du 1 , Mathias Steinacher 1 , Camelia Borca 2 , Thomas Huthwelker 2 , Anna Murello 3 , Francesco Stellacci 3 , Esther Amstad 1
Affiliation
|
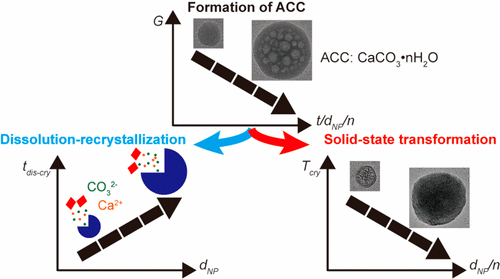
Calcium carbonate (CaCO3) is one of the most abundant biominerals that is prevalent in rocks and often used as a structural material in marine animals. Many of these natural CaCO3-based materials display excellent mechanical properties that are difficult to reproduce by man-made counterparts. This difficulty arises from the incomplete understanding of the influence of processing conditions on the structure and composition of CaCO3. To gain a better understanding of the evolution of the structure and composition of amorphous CaCO3 (ACC) particles during early stages, we introduce a new, organic solvent-free method that quenches this process with a high temporal resolution. We produce ACC particles inside small airborne drops that are formed with a microfluidic spray-dryer. These drops dry within 100 ms to 10 s and thereby arrest the formation of CaCO3 particles on that time scale. Using the microfluidic spray-dryer, we demonstrate that the amount of mobile water contained in ACC particles increases with increasing formation time and hence with increasing particle size. As a result of the higher concentration of mobile water, larger particles are less stable against temperature-induced solid-state crystallization and electron beam-induced decomposition than smaller counterparts. The amount of mobile water contained in ACC can be substantially reduced, and hence their kinetic stability against solid-state transformations increased, if certain organic additives, such as poly(acrylic acid) (PAA), are incorporated. These insights might open up new opportunities to fabricate biomimetic CaCO3-based materials with tunable structures and hence with properties that can be adapted to the needs of specific applications.
中文翻译:

无定形碳酸钙:形成时间对其水化程度和稳定性的影响
碳酸钙 (CaCO3) 是最丰富的生物矿物之一,在岩石中普遍存在,通常用作海洋动物的结构材料。许多这些天然 CaCO3 基材料显示出人造对应物难以复制的优异机械性能。这种困难源于对加工条件对 CaCO3 结构和组成影响的不完全理解。为了在早期阶段更好地了解无定形 CaCO3 (ACC) 颗粒的结构和组成的演变,我们引入了一种新的无有机溶剂方法,该方法以高时间分辨率淬灭该过程。我们在用微流体喷雾干燥器形成的小气滴内产生 ACC 颗粒。这些液滴会在 100 毫秒到 10 秒内干燥,从而在该时间范围内阻止 CaCO3 颗粒的形成。使用微流体喷雾干燥器,我们证明了 ACC 颗粒中包含的流动水量随着形成时间的增加而增加,因此随着粒径的增加而增加。由于流动水的浓度较高,与较小的对应物相比,较大的颗粒对温度诱导的固态结晶和电子束诱导的分解的稳定性较差。如果加入某些有机添加剂,例如聚(丙烯酸)(PAA),则 ACC 中所含的流动水量可以显着减少,因此它们对固态转变的动力学稳定性会增加。
更新日期:2018-09-28
中文翻译:

无定形碳酸钙:形成时间对其水化程度和稳定性的影响
碳酸钙 (CaCO3) 是最丰富的生物矿物之一,在岩石中普遍存在,通常用作海洋动物的结构材料。许多这些天然 CaCO3 基材料显示出人造对应物难以复制的优异机械性能。这种困难源于对加工条件对 CaCO3 结构和组成影响的不完全理解。为了在早期阶段更好地了解无定形 CaCO3 (ACC) 颗粒的结构和组成的演变,我们引入了一种新的无有机溶剂方法,该方法以高时间分辨率淬灭该过程。我们在用微流体喷雾干燥器形成的小气滴内产生 ACC 颗粒。这些液滴会在 100 毫秒到 10 秒内干燥,从而在该时间范围内阻止 CaCO3 颗粒的形成。使用微流体喷雾干燥器,我们证明了 ACC 颗粒中包含的流动水量随着形成时间的增加而增加,因此随着粒径的增加而增加。由于流动水的浓度较高,与较小的对应物相比,较大的颗粒对温度诱导的固态结晶和电子束诱导的分解的稳定性较差。如果加入某些有机添加剂,例如聚(丙烯酸)(PAA),则 ACC 中所含的流动水量可以显着减少,因此它们对固态转变的动力学稳定性会增加。































 京公网安备 11010802027423号
京公网安备 11010802027423号