Tetrahedron ( IF 2.1 ) Pub Date : 2018-09-28 , DOI: 10.1016/j.tet.2018.09.052 Shekhar Putta , Annem Mallikarjun Reddy , Gurrala Sheelu , B.V. Subba Reddy , Thenkrishnan Kumaraguru
|
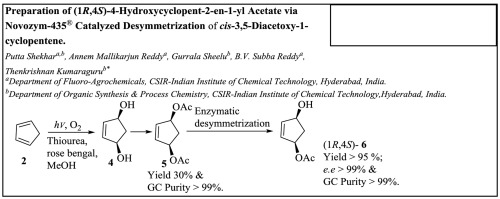
Photooxidation of cyclopentadiene has been carried out in methanol using white light of LED lamp, rose bengal as photo initiator, and compressed air at 0 °C. Under conditions of [thiourea] ≫ [cyclopentadiene], the consumption of thiourea follows a pseudo-first-order reaction kinetics with half life of 75 ± 10 min; corr. coeff. r = 0.989. Slow addition of the monomer and maintaining excess thiourea concentration in reaction mass improves the yield. cis-3,5-Dihydroxy-1-cyclopentene is acetylated without isolation to obtain cis-3,5-Diacetoxy-1-cyclopentene of high purity (>99%) with overall isolated yield of 30%. Desymmetrization of the diacetate to (1R,4S)-4-hydroxycyclopent-2-en-1-yl acetate has been carried out via enzymatic transesterification with methanol in methyl tert-butyl ether (MTBE) at 5 °C using Novozym-435®. The enantiomerically pure monoacetate (e.e. >99%) was obtained in 95% isolated yield. The recovered enzyme was reused for more than 10 times without loss in yield and selectivity. The entire protocol does not require purification of final product by chromatography.
中文翻译:

的(1制备- [R,4小号)-4-羟基环戊-2-烯-1-基乙酸酯的Novozym经由-435 ®的催化desymmetrization顺-3,5-二乙酰氧基-1-环戊烯
环戊二烯的光氧化反应是在甲醇中使用LED灯的白光,玫瑰红作为光引发剂和压缩空气在0°C进行的。在[硫脲]≫ [环戊二烯]的条件下,硫脲的消耗遵循拟一级反应动力学,半衰期为75±10分钟;更正 效率 r = 0.989。缓慢加入单体并保持反应物料中过量的硫脲浓度可提高产率。不分离地将顺式-3,5-二羟基-1-环戊烯乙酰化,得到高纯度(> 99%)的顺式-3,5-二乙酰氧基-1-环戊烯,总分离产率为30%。双乙酸盐的不对称化为(1 R,4 S基)-4-羟基环戊-2-烯-1-基乙酸酯已经经由进行酶促酯交换反应用甲醇在甲基叔丁基醚(MTBE)在5℃下使用的Novozym-435 ®。得到对映体纯的单乙酸酯(ee> 99%),分离产率为95%。回收的酶可重复使用10次以上,而不会损失收率和选择性。整个方案不需要通过色谱法纯化最终产物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号