当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Oxygen Vacancies on Oxygen Evolution Reaction Activity: β-Ga2O3 as a Case Study
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.chemmater.8b03015 Taifeng Liu 1 , Zhaochi Feng 2 , Qiuye Li 1 , Jianjun Yang 1 , Can Li 2 , Michel Dupuis 2, 3
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.chemmater.8b03015 Taifeng Liu 1 , Zhaochi Feng 2 , Qiuye Li 1 , Jianjun Yang 1 , Can Li 2 , Michel Dupuis 2, 3
Affiliation
|
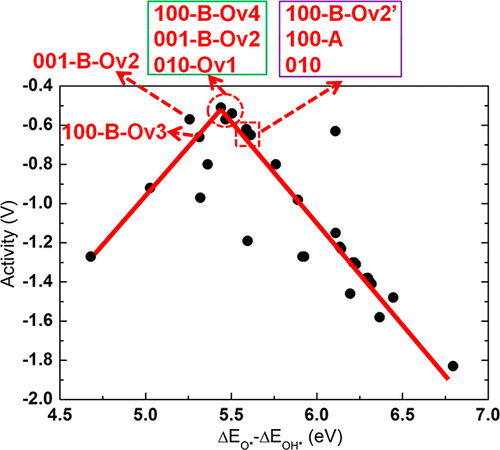
Neutral oxygen vacancies (Ovʼs) in semiconductor oxides give rise to excess electrons that have the potential to affect the binding of adsorbates to the surface through surface-to-adsorbate charge transfer, and, as a result, to alter the overpotential (OP) of reactions on oxygen-deficient materials compared to stoichiometric materials. We report a systematic computational investigation of the effects of Ovʼs on the oxygen evolution reaction (OER) overpotential for β-Ga2O3, a d10 semiconductor that has been shown to exhibit high activity for water splitting. We investigated 18 β-Ga2O3 surfaces/slabs, with and without Ovʼs and observed a clear dependence of OER activity on Ovʼs. A general finding emerged that the excess electrons associated with Ovʼs are found to participate in charge transfer to OER intermediates, making their bonds to the surface more ionic and stronger, depending on the amount of charge transfer. The OER reaction step free energies are significantly affected and the ensuing overpotentials are altered. The amount of charge transfer varies with the types of intermediates (OH*, dangling O*, surface-bound peroxo O*, and dangling OOH*), their open valencies, and their electronegativity. The work function and the position of the gap states of the excess electrons in the band gap are found to be useful descriptors of whether and how much Ov-induced charge transfer may occur and affect the overpotential. However, it was also found that the chemical environment of the O atom where the vacancy was created, may have a negating effect on the general observation. Specifically some Ov structures underwent a strong relaxation to form Ga–Ga bonds, trapping the vacancy electrons, and preventing them to engage in charge transfer. Oxygen vacancies are common defects in photocatalyst materials so that our investigation can provide guiding principles for designing efficient photocatalysts.
中文翻译:

上析氧反应活性氧空位的作用:的β-Ga 2 ö 3为典型案例
半导体氧化物中的中性氧空位(Ovʼs)产生过量的电子,这些电子有可能通过表面到吸附物的电荷转移影响吸附物与表面的结合,并因此改变金属的超电势(OP)。与化学计量材料相比,对缺氧材料的反应。我们报告的Ov's对析氧反应的影响的系统的计算调查(OER)超电势的β-Ga 2 ö 3,一个d 10已被证明表现出的水分解活性高的半导体。我们研究了18的β-Ga 2 ö 3在有或没有Ovʼs的表面/平板上,观察到OER活性对Ovʼs有明显的依赖性。一个普遍的发现是,发现与Ovʼs相关的过量电子会参与电荷转移到OER中间体,从而使它们与表面的键更具离子性和强度,这取决于电荷转移的量。OER反应步骤的自由能受到显着影响,随后的过电势也发生了变化。电荷转移的量随中间体的类型(OH *,悬挂的O *,表面结合的过氧O *和悬挂的OOH *),开环价和电负性而变化。发现功函数和带隙中多余电子的间隙状态的位置是是否可能发生以及由多少Ov引起的电荷转移并影响过电势的有用描述。然而,还发现产生空位的O原子的化学环境可能对一般观察结果有负面影响。具体来说,某些Ov结构经历了强烈的弛豫,形成了Ga-Ga键,从而捕获了空位电子,并阻止了它们参与电荷转移。氧空位是光催化剂材料中的常见缺陷,因此我们的研究可以为设计有效的光催化剂提供指导原则。
更新日期:2018-09-27
中文翻译:

上析氧反应活性氧空位的作用:的β-Ga 2 ö 3为典型案例
半导体氧化物中的中性氧空位(Ovʼs)产生过量的电子,这些电子有可能通过表面到吸附物的电荷转移影响吸附物与表面的结合,并因此改变金属的超电势(OP)。与化学计量材料相比,对缺氧材料的反应。我们报告的Ov's对析氧反应的影响的系统的计算调查(OER)超电势的β-Ga 2 ö 3,一个d 10已被证明表现出的水分解活性高的半导体。我们研究了18的β-Ga 2 ö 3在有或没有Ovʼs的表面/平板上,观察到OER活性对Ovʼs有明显的依赖性。一个普遍的发现是,发现与Ovʼs相关的过量电子会参与电荷转移到OER中间体,从而使它们与表面的键更具离子性和强度,这取决于电荷转移的量。OER反应步骤的自由能受到显着影响,随后的过电势也发生了变化。电荷转移的量随中间体的类型(OH *,悬挂的O *,表面结合的过氧O *和悬挂的OOH *),开环价和电负性而变化。发现功函数和带隙中多余电子的间隙状态的位置是是否可能发生以及由多少Ov引起的电荷转移并影响过电势的有用描述。然而,还发现产生空位的O原子的化学环境可能对一般观察结果有负面影响。具体来说,某些Ov结构经历了强烈的弛豫,形成了Ga-Ga键,从而捕获了空位电子,并阻止了它们参与电荷转移。氧空位是光催化剂材料中的常见缺陷,因此我们的研究可以为设计有效的光催化剂提供指导原则。































 京公网安备 11010802027423号
京公网安备 11010802027423号