当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Selective Conversion of Azido Groups at Carbonyl α-Positions to Diazo Groups in Diazido and Triazido Compounds
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.joc.8b02074 Taiki Yokoi 1 , Hiroki Tanimoto 1 , Tomomi Ueda 1 , Tsumoru Morimoto 1 , Kiyomi Kakiuchi 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.joc.8b02074 Taiki Yokoi 1 , Hiroki Tanimoto 1 , Tomomi Ueda 1 , Tsumoru Morimoto 1 , Kiyomi Kakiuchi 1
Affiliation
|
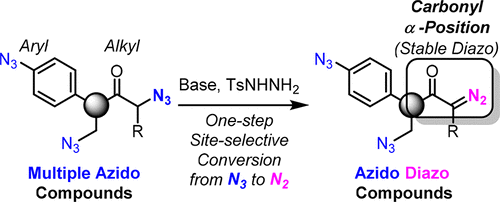
This paper reports on the selective conversion of alkyl azido groups at the carbonyl α-position to diazo compounds. Through β-elimination of dinitrogen, followed by hydrazone formation/decomposition, α-azidocarbonyl moieties were transformed into α-diazo carbonyl groups in one step. As these reaction conditions do not involve aryl or general alkyl azides, site-selective conversions of di- and triazides were achieved. Through this method, the successive site-selective conjugation of the triazido molecule with three different components is demonstrated.
中文翻译:

羰基α位置的叠氮基位点选择性转化为重氮基和三叠氮化合物中的重氮基团
本文报道了羰基α位烷基叠氮基选择性转化为重氮化合物的报道。通过β-消除二氮,然后形成/分解,一步将α-叠氮羰基部分转化为α-重氮羰基。由于这些反应条件不涉及芳基或普通烷基叠氮化物,因此实现了二叠氮化物和三叠氮化物的位点选择性转化。通过这种方法,证明了具有三个不同组分的三叠氮分子的连续位点选择性缀合。
更新日期:2018-09-27
中文翻译:

羰基α位置的叠氮基位点选择性转化为重氮基和三叠氮化合物中的重氮基团
本文报道了羰基α位烷基叠氮基选择性转化为重氮化合物的报道。通过β-消除二氮,然后形成/分解,一步将α-叠氮羰基部分转化为α-重氮羰基。由于这些反应条件不涉及芳基或普通烷基叠氮化物,因此实现了二叠氮化物和三叠氮化物的位点选择性转化。通过这种方法,证明了具有三个不同组分的三叠氮分子的连续位点选择性缀合。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号