当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic Investigation for Oxygen Reduction and Evolution Reactions with Tetrathiafulvalene as a Redox Mediator in Li–O2 Battery
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-09 00:00:00 , DOI: 10.1021/acs.jpcc.5b11692 Yu Qiao 1 , Shen Ye 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-09 00:00:00 , DOI: 10.1021/acs.jpcc.5b11692 Yu Qiao 1 , Shen Ye 1
Affiliation
|
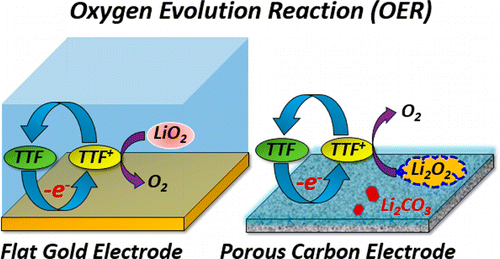
To develop a lithium–oxygen (Li–O2) battery with a high specific energy, the electrochemical oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) have been systematically investigated on gold and porous carbon electrodes in a DMSO-based electrolyte solution containing tetrathiafulvalene (TTF) as a redox mediator by using in situ UV–vis absorption spectroscopy and surface-enhanced Raman vibrational spectroscopy (SERS) in combination with ex situ infrared and Raman spectroscopies. Our results demonstrate that TTF definitely reduces the overpotential for the OER process and restrains the decomposition of the carbon electrode and solvent during the OER, while the functionality of the redox mediator can change with the electrode materials and morphologies. No free TTF+ was observed in solution during a round-trip galvanostatic ORR/OER cycle on the gold electrode surface. The electrochemically generated TTF+ was mainly consumed by the oxidative decomposition of lithium superoxide (LiO2) in solution but not lithium peroxide (Li2O2) on the electrode surface formed during the ORR, different from that previously proposed. On the other hand, Li2O2 and Li2CO3 were observed during the ORR on the porous carbon electrode surface both in the TTF-free and TTF-containing solutions. The TTF+ can mediate the oxidation of the Li2O2 during the OER. Furthermore, the accumulation of free TTF+ was observed in solution during a round-trip galvanostatic ORR/OER cycle on the porous carbon electrode. The amount of the excess TTF+ in solution well corresponds to that of the byproduct of Li2CO3 formed on the carbon electrode surface. TTF is unlikely to work as an ideal redox mediator at the porous carbon electrode as expected. A better understanding for the mechanism and surface reactions for the ORR/OER cycle on these electrodes has been achieved by the present study.
中文翻译:

四氧化硫富瓦烯作为氧化还原介体在Li-O 2电池中进行氧还原和放出反应的光谱研究
为了开发具有高比能量的锂氧(Li–O 2)电池,已经在基于DMSO的电解液中的金和多孔碳电极上系统地研究了电化学氧还原反应(ORR)和氧释放反应(OER)。原位紫外-可见吸收光谱和表面增强拉曼振动光谱(SERS)结合非原位结合,以四硫富瓦烯(TTF)为氧化还原介质的溶液红外和拉曼光谱。我们的结果表明,TTF无疑会降低OER过程的过电势,并抑制OER过程中碳电极和溶剂的分解,而氧化还原介体的功能会随电极材料和形态而变化。在金电极表面上的往返恒电流ORR / OER循环过程中,在溶液中未观察到游离的TTF +。不同于先前提出的,电化学产生的TTF +主要通过溶液中的过氧化锂(LiO 2)在溶液中的氧化分解而被消耗,而不被在ORR形成的电极表面上的过氧化锂(Li 2 O 2)氧化消耗。另一方面,李2在无TTF溶液和含TTF溶液中,在多孔碳电极表面的ORR过程中均观察到O 2和Li 2 CO 3。在OER期间,TTF +可以介导Li 2 O 2的氧化。此外,在多孔碳电极上的往返恒电流ORR / OER循环过程中,观察到溶液中游离TTF +的积累。溶液中过量的TTF +的量对应于Li 2 CO 3的副产物的量。在碳电极表面上形成。如预期的那样,TTF不太可能在多孔碳电极上充当理想的氧化还原介体。通过本研究已经更好地理解了这些电极上ORR / OER循环的机理和表面反应。
更新日期:2016-02-09
中文翻译:

四氧化硫富瓦烯作为氧化还原介体在Li-O 2电池中进行氧还原和放出反应的光谱研究
为了开发具有高比能量的锂氧(Li–O 2)电池,已经在基于DMSO的电解液中的金和多孔碳电极上系统地研究了电化学氧还原反应(ORR)和氧释放反应(OER)。原位紫外-可见吸收光谱和表面增强拉曼振动光谱(SERS)结合非原位结合,以四硫富瓦烯(TTF)为氧化还原介质的溶液红外和拉曼光谱。我们的结果表明,TTF无疑会降低OER过程的过电势,并抑制OER过程中碳电极和溶剂的分解,而氧化还原介体的功能会随电极材料和形态而变化。在金电极表面上的往返恒电流ORR / OER循环过程中,在溶液中未观察到游离的TTF +。不同于先前提出的,电化学产生的TTF +主要通过溶液中的过氧化锂(LiO 2)在溶液中的氧化分解而被消耗,而不被在ORR形成的电极表面上的过氧化锂(Li 2 O 2)氧化消耗。另一方面,李2在无TTF溶液和含TTF溶液中,在多孔碳电极表面的ORR过程中均观察到O 2和Li 2 CO 3。在OER期间,TTF +可以介导Li 2 O 2的氧化。此外,在多孔碳电极上的往返恒电流ORR / OER循环过程中,观察到溶液中游离TTF +的积累。溶液中过量的TTF +的量对应于Li 2 CO 3的副产物的量。在碳电极表面上形成。如预期的那样,TTF不太可能在多孔碳电极上充当理想的氧化还原介体。通过本研究已经更好地理解了这些电极上ORR / OER循环的机理和表面反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号