当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of the Dependence of the Flory–Huggins Interaction Parameter on Temperature and Composition in a Drug–Polymer System
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00797 Catherine B. Potter 1 , Mark T. Davis 1 , Ahmad B. Albadarin 1 , Gavin M. Walker 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00797 Catherine B. Potter 1 , Mark T. Davis 1 , Ahmad B. Albadarin 1 , Gavin M. Walker 1
Affiliation
|
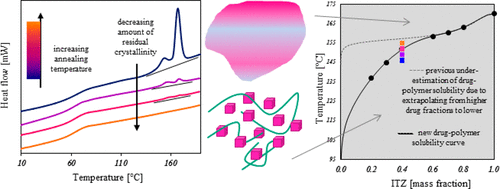
The Flory–Huggins (F–H) solubility equation has been widely used to describe the solubility of a small-molecule drug in a polymeric carrier and thus determine the design space available for formulating a stable amorphous solid dispersion. The F–H interaction parameter (χ) describes the thermodynamic properties of drug–polymer solutions and accounts for any enthalpic and entropic changes in solubility. Many studies have found that for a limited compositional range, χ varies proportionally to the inverse of the melting temperature of the drug. We explored this relationship using a highly sensitive DSC technique to detect remaining residual crystalline active pharmaceutical ingredients (APIs) following annealing of ball milled mixtures of crystalline itraconazole (ITZ) and either Soluplus or hydroxypropyl methylcellulose phthalate (HPMCP) at temperatures near the estimated solubility curve. Depending on the experimental approach taken, the measurement of drug–polymer solubility can be restricted to mixtures with a high proportion of drug, but in this study, solubility was experimentally determined for mixtures with API content as low as 10 wt %. Results suggest that the proposed linear relationship does not extend to compositions with smaller amounts of API, instead indicating that χ was both temperature- and composition-dependent for the systems studied. The feasibility of this technique to measure interactions in a ternary system containing itraconazole and both polymers was also determined; ITZ–HPMCP exhibited the most favorable values of χ, while ITZ–Soluplus and ITZ–Soluplus–HPMCP demonstrated similar interaction parameters.
中文翻译:

药物-聚合物系统中弗洛里-哈金斯相互作用参数对温度和组成的依赖性研究
Flory-Huggins(F-H)溶解度方程已被广泛用于描述小分子药物在聚合物载体中的溶解度,从而确定了可用于配制稳定的无定形固体分散体的设计空间。F–H相互作用参数(χ)描述了药物-聚合物溶液的热力学性质,并说明了溶解度的任何焓变和熵变。许多研究发现,在有限的组成范围内,χ与药物的解链温度成反比。我们使用高灵敏度的DSC技术探索了这种关系,以在结晶估计的伊曲康唑(ITZ)和Soluplus或羟丙基甲基纤维素邻苯二甲酸酯(HPMCP)的球磨混合物退火后在估计的溶解度曲线附近的温度下检测残留的残余结晶活性药物成分。根据所采用的实验方法,可以将药物-聚合物溶解度的测量限制在药物比例较高的混合物中,但在本研究中,通过实验确定了API含量低至10 wt%的混合物的溶解度。结果表明,所提出的线性关系不会扩展到使用较少量API的成分,而是表明对于所研究的系统,χ既依赖于温度,又依赖于成分。还确定了该技术在包含伊曲康唑和两种聚合物的三元体系中测量相互作用的可行性。ITZ–HPMCP表现出最有利的χ值,而ITZ–Soluplus和ITZ–Soluplus–HPMCP表现出相似的相互作用参数。
更新日期:2018-09-27
中文翻译:

药物-聚合物系统中弗洛里-哈金斯相互作用参数对温度和组成的依赖性研究
Flory-Huggins(F-H)溶解度方程已被广泛用于描述小分子药物在聚合物载体中的溶解度,从而确定了可用于配制稳定的无定形固体分散体的设计空间。F–H相互作用参数(χ)描述了药物-聚合物溶液的热力学性质,并说明了溶解度的任何焓变和熵变。许多研究发现,在有限的组成范围内,χ与药物的解链温度成反比。我们使用高灵敏度的DSC技术探索了这种关系,以在结晶估计的伊曲康唑(ITZ)和Soluplus或羟丙基甲基纤维素邻苯二甲酸酯(HPMCP)的球磨混合物退火后在估计的溶解度曲线附近的温度下检测残留的残余结晶活性药物成分。根据所采用的实验方法,可以将药物-聚合物溶解度的测量限制在药物比例较高的混合物中,但在本研究中,通过实验确定了API含量低至10 wt%的混合物的溶解度。结果表明,所提出的线性关系不会扩展到使用较少量API的成分,而是表明对于所研究的系统,χ既依赖于温度,又依赖于成分。还确定了该技术在包含伊曲康唑和两种聚合物的三元体系中测量相互作用的可行性。ITZ–HPMCP表现出最有利的χ值,而ITZ–Soluplus和ITZ–Soluplus–HPMCP表现出相似的相互作用参数。






























 京公网安备 11010802027423号
京公网安备 11010802027423号