当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation and Aggregation of Lead Phosphate Particles: Implications for Lead Immobilization in Water Supply Systems
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-10-09 , DOI: 10.1021/acs.est.8b02788 Juntao Zhao 1 , Daniel E. Giammar 2 , Jill D. Pasteris 3 , Chong Dai 1 , Yeunook Bae 2 , Yandi Hu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-10-09 , DOI: 10.1021/acs.est.8b02788 Juntao Zhao 1 , Daniel E. Giammar 2 , Jill D. Pasteris 3 , Chong Dai 1 , Yeunook Bae 2 , Yandi Hu 1
Affiliation
|
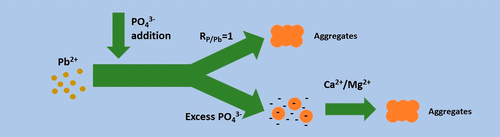
Phosphate is commonly added to drinking water to inhibit lead release from lead service lines and lead-containing materials in premise plumbing. Phosphate addition promotes the formation of lead phosphate particles, and their aggregation behaviors may affect their transport in pipes. Here, lead phosphate formation and aggregation were studied under varied aqueous conditions typical of water supply systems. Under high aqueous PO4/Pb molar ratios (>1), phosphate adsorption made the particles more negatively charged. Therefore, enhanced stability of lead phosphate particles was observed, suggesting that although addition of excess phosphate can lower the dissolved lead concentrations in tap water, it may increase concentrations of particulate lead. Adsorption of divalent cations (Ca2+ and Mg2+) onto lead phosphate particles neutralized their negative surface charges and promoted their aggregation at pH 7, indicating that phosphate addition for lead immobilization may be more efficient in harder waters. The presence of natural organic matter (NOM, ≥ 0.05 mg C/L humic acid and ≥ 0.5 mg C/L fulvic acid) retarded particle aggregation at pH 7. Consequently, removal of organic carbon during water treatment to lower the formation of disinfection-byproducts (DBPs) may have the additional benefit of minimizing the mobility of lead-containing particles. This study provided insight into fundamental mechanisms controlling lead phosphate aggregation. Such understanding is helpful to understand the observed trends of total lead in water after phosphate addition in both field and pilot-scale lead pipe studies. Also, it can help optimize lead immobilization by better controlling the water chemistry during phosphate addition.
中文翻译:

磷酸铅颗粒的形成和聚集:对供水系统中铅固定化的意义
通常将磷酸盐添加到饮用水中,以抑制铅从自来水管道和室内管道中的含铅材料中释放出来。添加磷酸盐会促进磷酸铅颗粒的形成,并且它们的聚集行为可能会影响其在管道中的运输。在此,研究了在供水系统典型的各种含水条件下磷酸铅的形成和聚集。在高含水PO 4 / Pb摩尔比(> 1)下,磷酸盐的吸附使颗粒带更多的负电荷。因此,观察到磷酸铅颗粒的稳定性增强,这表明尽管添加过量的磷酸盐可以降低自来水中溶解的铅浓度,但它可能会增加颗粒状铅的浓度。吸附二价阳离子(Ca 2+和镁2+)到磷酸铅上的粒子中和了它们的负表面电荷,并促进了它们在pH 7时的聚集,这表明在较硬的水中添加磷酸盐来固定铅可能更有效。天然有机物(NOM,≥0.05 mg C / L腐殖酸和≥0.5 mg C / L黄腐酸)的存在会在pH 7时阻碍颗粒聚集。因此,在水处理过程中去除有机碳可减少消毒的形成,副产物(DBP)可能具有将含铅颗粒的迁移率降至最低的额外好处。该研究提供了控制磷酸铅聚集的基本机制的见解。这种理解有助于理解在现场和中试规模的铅管研究中磷酸盐添加后水中总铅的观测趋势。还,
更新日期:2018-10-09
中文翻译:

磷酸铅颗粒的形成和聚集:对供水系统中铅固定化的意义
通常将磷酸盐添加到饮用水中,以抑制铅从自来水管道和室内管道中的含铅材料中释放出来。添加磷酸盐会促进磷酸铅颗粒的形成,并且它们的聚集行为可能会影响其在管道中的运输。在此,研究了在供水系统典型的各种含水条件下磷酸铅的形成和聚集。在高含水PO 4 / Pb摩尔比(> 1)下,磷酸盐的吸附使颗粒带更多的负电荷。因此,观察到磷酸铅颗粒的稳定性增强,这表明尽管添加过量的磷酸盐可以降低自来水中溶解的铅浓度,但它可能会增加颗粒状铅的浓度。吸附二价阳离子(Ca 2+和镁2+)到磷酸铅上的粒子中和了它们的负表面电荷,并促进了它们在pH 7时的聚集,这表明在较硬的水中添加磷酸盐来固定铅可能更有效。天然有机物(NOM,≥0.05 mg C / L腐殖酸和≥0.5 mg C / L黄腐酸)的存在会在pH 7时阻碍颗粒聚集。因此,在水处理过程中去除有机碳可减少消毒的形成,副产物(DBP)可能具有将含铅颗粒的迁移率降至最低的额外好处。该研究提供了控制磷酸铅聚集的基本机制的见解。这种理解有助于理解在现场和中试规模的铅管研究中磷酸盐添加后水中总铅的观测趋势。还,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号