Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-09-25 , DOI: 10.1016/j.bioorg.2018.09.034 Khaled R.A. Abdellatif , Wael A.A. Fadaly , Gehan M. Kamel , Yaseen A.M.M. Elshaier , Mohammed A. El-Magd
|
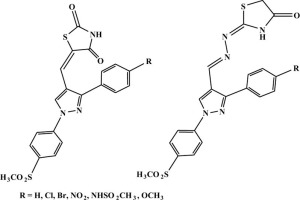
Nowadays, diabetes and its associated inflammatory complications are important public health problems worldwide. Market limitations of drugs with dual actions as anti-inflammatory (AI) and anti-diabetic have been led to a temptation for focusing on the discovery and development of new compounds with potential AI and anti-diabetic activities. Herein, we synthesized two new series containing pyrazole ring with vicinal diaryl rings as selective COX-2 moiety and thiazolidindione (series 12a-f) or thiazolidinone (series 13a-f) as anti-diabetic moiety and the two moieties were linked together with methylene or methylenehydrazone functionality. The two series were evaluated for their COX inhibition, AI activity and ulcerogenic liability and for the anti-diabetic activity; 12a-f and 13a-f were assessed in vitro against α-glucosidase, β- glucosidase, in vivo hypoglycemic activity (one day and 15 days studies) in addition to PPARγ activation study. Four compounds (12c, 12f, 13b and 13f) had higher COX-2 S.I. (8.69–9.26) than the COX-2 selective drug celecoxib (COX-2 S.I. = 8.60) and showed the highest AI activities and the lowest ulcerogenicity than other derivatives. Also, two thiazolidindione derivatives 12e and 12f and two thiazolidinone derivatives 13b and 13c showed higher inhibitory activities against α- and β-glucosidase (% inhibitory activity = 62.15, 55.30, 65.37, 59.08 for α-glucosidase and 57.42, 60.07, 58.19, 66.90 for β-glucosidase respectively) than reference compounds (acarbose with % inhibitory activity = 49.50 for α-glucosidase and d-saccharic acid 1,4-lactone monohydrate with % inhibitory activity = 53.42 for β-glucosidase) and also showed good PPAR-γ activation and good hypoglycemic effect in comparison to pioglitazone and rosiglitazone. Moreover, Shape comparison and docking studies were carried out to understand their interaction and similarity with standard drugs.
中文翻译:

含吡唑核心作为潜在抗糖尿病PPAR-γ激动剂和抗炎COX-2选择性抑制剂的噻唑烷衍生物的设计,合成,建模研究和生物学评估
如今,糖尿病及其相关的炎症并发症已成为全球范围内重要的公共卫生问题。具有抗炎(AI)和抗糖尿病双重作用的药物的市场局限性导致人们倾向于专注于发现和开发具有潜在AI和抗糖尿病活性的新化合物。在本文中,我们合成了两个新的系列,其中含有带有邻二芳基环作为选择性COX-2部分的吡唑环和噻唑烷二酮(12a-f系列)或噻唑烷酮(13a-f系列)作为抗糖尿病部分,并且两个部分与亚甲基连接在一起或的功能。对这两个系列的COX抑制,AI活性和致溃疡性以及抗糖尿病活性进行了评估。12a-f除了PPARγ激活研究外,还评估了α-葡萄糖苷酶和13a-f在体外的抗α-葡萄糖苷酶,β-葡萄糖苷酶,体内降血糖活性(研究1天和15天)。四种化合物(12c,12f,13b和13f)的COX-2 SI值(8.69–9.26)高于COX-2选择性药物塞来昔布(COX-2 SI = 8.60),并且具有最高的AI活性和最低的致溃疡性衍生品。另外,两种噻唑烷二酮衍生物12e和12f以及两种噻唑烷酮衍生物13b和13c显示出对α-的更高的抑制活性。和β-葡萄糖苷酶(对α-葡萄糖苷酶的抑制活性分别为62.15、55.30、65.37、59.08和对β-葡萄糖苷酶的抑制活性分别为57.42、60.07、58.19、66.90)相对于参考化合物(对%糖苷酶的抑制活性为49.50的α-葡萄糖苷酶和与吡格列酮和罗格列酮相比,d-糖酸1,4-内酯一水合物对β-葡萄糖苷酶的%抑制活性= 53.42),并且还显示出良好的PPAR-γ活化和良好的降血糖作用。此外,进行了形状比较和对接研究,以了解它们与标准药物的相互作用和相似性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号