Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2018-09-24 , DOI: 10.1016/j.apcatb.2018.09.058 Junbo Zhong , Yukun Zhao , Liyong Ding , Hongwei Ji , Wanhong Ma , Chuncheng Chen , Jincai Zhao
|
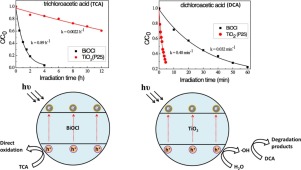
Although all the semiconductor-based photocatalysis is initiated by photoinduced conduction band electron and valence band hole, the reaction pathway and activity of the electron and hole would be largely dependent on the type of the photocatalyst. It is essential to distinguish and understand the photocatalyst-dependence of a specific reaction. In the present study, we compared the photocatalytic behaviors of BiOCl and TiO2 for the degradation of different pollutants including perfluorooctanoic acid (PFOA), chloroacetic acids and benzoic acid. We found that the decompositions of perhalocarboxylate acids (PHCAs) such as PFOA and trichloroacetic acid (TCA) were much rapider over BiOCl than on the TiO2 (commercial P25). The surface-area- normalized rate constants for the oxidation of TCA have five orders of magnitude of difference between these two systems. By contrast, the degradation rates of OH-sensitive organic pollutants such as dichloroacetic acid, monochloroacetic acid and benzoic acid were much higher on the TiO2. Moreover, much more meta-substituted hydroxylated intermediate was observed during the photocatalytic oxidation of benzoic acid on BiOCl. In addition, PFOA and TCA were degraded efficiently in the BiOCl system even in the presence of other labile organic compounds (such as acetic acid). All the experimental results definitely indicate that BiOCl photocatalyst prefers to directly oxidize the PHCAs and benzoic acid by the hole transfer, while TiO2 tends to oxidize the solvent water molecule to
OH radical. The mechanism underlying on the different activity of BiOCl and TiO2 are further discussed.
中文翻译:

BiOCl和TiO 2的相反光催化氧化行为:直接空穴转移与间接
尽管所有基于半导体的光催化都是由光诱导的导带电子和价带空穴引发的,但是电子和空穴的反应途径和活性在很大程度上取决于光催化剂的类型。区分和理解特定反应的光催化剂依赖性至关重要。在本研究中,我们比较了BiOCl和TiO 2对包括全氟辛酸(PFOA),氯乙酸和苯甲酸在内的不同污染物的降解的光催化行为。我们发现,全氟化羧酸(PHCA)(如PFOA和三氯乙酸(TCA))在BiOCl上的分解要比在TiO 2上快得多。(商业P25)。TCA氧化的表面面积归一化速率常数在这两个系统之间有五个数量级的差异。相反,在TiO 2上,对OH敏感的有机污染物(如二氯乙酸,一氯乙酸和苯甲酸)的降解速率要高得多。而且,在BiOCl上苯甲酸的光催化氧化过程中观察到更多的间位取代的羟基化中间体。此外,即使在存在其他不稳定的有机化合物(例如乙酸)的情况下,BiOCl系统中的PFOA和TCA也会被有效降解。所有实验结果明确表明,BiOCl光催化剂更喜欢通过空穴转移直接氧化PHCA和苯甲酸,而TiO 2倾向于将溶剂水分子氧化成
OH自由基。进一步讨论了基于BiOCl和TiO 2的不同活性的机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号