当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Sulfide-Catalyzed Diastereoselective [4+1] Annulations of ortho-Quinone Methides and Bromides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acs.joc.8b02189 Yan-Qing Liu 1 , Qing-Zhu Li 2 , Hong-Ping Zhu 1, 2 , Xin Feng 2 , Cheng Peng 1 , Wei Huang 1 , Jun-Long Li 2 , Bo Han 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acs.joc.8b02189 Yan-Qing Liu 1 , Qing-Zhu Li 2 , Hong-Ping Zhu 1, 2 , Xin Feng 2 , Cheng Peng 1 , Wei Huang 1 , Jun-Long Li 2 , Bo Han 1
Affiliation
|
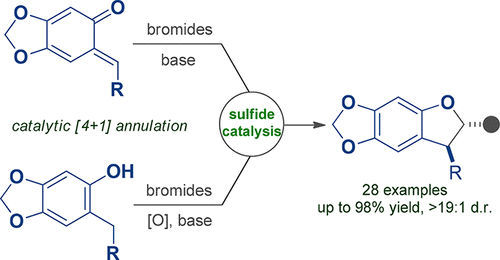
Direct sulfide organocatalysis is an emerging topic in research on synthetic chemistry. Here, an unprecedented sulfide-catalyzed diastereoselective [4+1] annulation of (in situ generated) ortho-quinone methides and bromides is reported. Notably, the robustness of such sulfide organocatalysis was demonstrated by performing the catalytic reaction under oxidative conditions without significantly affecting the reaction outcome. Various dihydrobenzofurans with diverse substituents were obtained with high isolated yields of up to 98% and remarkable diastereoselectivity (>19:1 dr in general).
中文翻译:

邻苯二甲腈和溴化物的直接硫化物催化的非对映选择性[4 + 1]环
直接硫化物有机催化是合成化学研究中的一个新兴课题。在此,报道了(原位生成的)邻苯醌甲基化物和溴化物的前所未有的硫化物催化的非对映选择性[4 + 1]环化反应。值得注意的是,通过在氧化条件下进行催化反应而不显着影响反应结果证明了这种硫化物有机催化的稳健性。获得了具有不同取代基的各种二氢苯并呋喃,具有高达98%的高分离收率和显着的非对映选择性(通常> 19:1 dr)。
更新日期:2018-09-24
中文翻译:

邻苯二甲腈和溴化物的直接硫化物催化的非对映选择性[4 + 1]环
直接硫化物有机催化是合成化学研究中的一个新兴课题。在此,报道了(原位生成的)邻苯醌甲基化物和溴化物的前所未有的硫化物催化的非对映选择性[4 + 1]环化反应。值得注意的是,通过在氧化条件下进行催化反应而不显着影响反应结果证明了这种硫化物有机催化的稳健性。获得了具有不同取代基的各种二氢苯并呋喃,具有高达98%的高分离收率和显着的非对映选择性(通常> 19:1 dr)。






































 京公网安备 11010802027423号
京公网安备 11010802027423号