当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Well-Defined Palladium(II)–NHC Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective N–C/O–C Cleavage
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1021/acs.accounts.8b00410 Shicheng Shi 1 , Steven P. Nolan 2, 3 , Michal Szostak 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1021/acs.accounts.8b00410 Shicheng Shi 1 , Steven P. Nolan 2, 3 , Michal Szostak 1
Affiliation
|
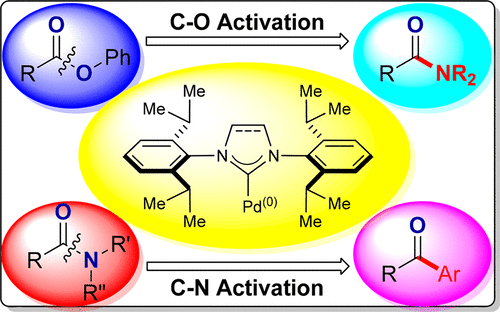
Transition-metal-catalyzed cross-coupling reactions represent a most powerful tool for the rapid construction of C–C and C–X bonds available to synthetic chemists. Recently, tremendous progress has been made in the burgeoning area of cross-coupling reactions of amides and esters enabled by regio- and chemoselective acyl C–X (X = N, O) cleavage using well-defined Pd(II)–NHC complexes. The use of N-heterocyclic carbenes as ligands in palladium-catalyzed cross-couplings permits reactions of amides and esters that were previously impossible using palladium or could be achieved only under harsh conditions. These reactions provide an attractive method to synthetic chemists to manipulate the traditionally inert amide and ester bonds with the broad cross-coupling generality inherent to palladium catalysis. Research in the area of cross-coupling of stable acyl electrophiles can be broadly categorized by the type of electrophile undergoing the cross-coupling. Recent studies have shown that cross-coupling of amides by transition-metal catalysis represents one of the most straightforward and wide-ranging ways of manipulating the classically inert amide bonds into generic acyl–metal intermediates that can be systematically exploited in cross-coupling reactions as a new paradigm in organic synthesis. The key to achieving high chemoselectivity of the process is control of amidic resonance (nN to πC═O* conjugation, rotation of ca. 15–20 kcal/mol in planar amides), enabling oxidative addition of the N–C amide bond to a metal in a rational and predictable manner. This mode of catalysis has been extended to C(acyl)–O cross-coupling reactions of aryl esters, where selective C–O bond cleavage is accomplished through a rational match of aryl ester electrophiles and nucleophilic metal catalysts. These two types of transition-metal-catalyzed cross-coupling reactions represent an attractive concept in synthetic chemistry because of the ubiquity of esters and amides as precursors in organic synthesis. Furthermore, the high stability of amides and esters provides unprecedented opportunities for orthogonal cross-coupling strategies in the presence of other electrophiles.
中文翻译:

定义明确的钯(II)-NHC预催化剂,用于通过选择性NC-OC / C裂解进行酰胺和酯的交叉偶联反应
过渡金属催化的交叉偶联反应是合成化学家可快速构建C–C和C–X键的最有力工具。最近,在利用明确定义的Pd(II)-NHC络合物进行区域和化学选择性酰基C–X(X = N,O)裂解的酰胺和酯的交叉偶联反应的新兴领域,已取得了巨大进展。在钯催化的交叉偶联中使用N-杂环卡宾作为配体可以使酰胺和酯发生反应,而以前使用钯是不可能的,或者只有在苛刻的条件下才能实现。这些反应为合成化学家提供了一种诱人的方法,可以以钯催化固有的广泛交叉偶联通用性来操作传统上惰性的酰胺和酯键。稳定酰基亲电试剂的交叉偶联领域的研究可以按经历交叉偶联的亲电试剂的类型大致分类。最近的研究表明,过渡金属催化的酰胺交叉偶联是将经典惰性酰胺键转化为通用的酰基金属中间体的最直接,最广泛的方法之一,可以在交叉偶联反应中系统地利用该中间体。有机合成的新范式。实现该过程的高化学选择性的关键是控制酰胺共振(n 最近的研究表明,过渡金属催化的酰胺交叉偶联是将经典惰性酰胺键转化为通用的酰基金属中间体的最直接,最广泛的方法之一,可以在交叉偶联反应中系统地利用该中间体。有机合成的新范式。实现该过程高化学选择性的关键是控制酰胺共振(n 最近的研究表明,过渡金属催化的酰胺交叉偶联是将经典惰性酰胺键转化为通用的酰基金属中间体的最直接,最广泛的方法之一,可以在交叉偶联反应中系统地利用该中间体。有机合成的新范式。实现该过程的高化学选择性的关键是控制酰胺共振(nN到πC═O *共轭,旋转约ca。在平面酰胺中为15–20 kcal / mol),从而能够以合理且可预测的方式将N–C酰胺键氧化加成到金属上。这种催化方式已扩展到芳基酯的C(酰基)-O交叉偶联反应,其中通过合理匹配芳基酯亲电子试剂和亲核金属催化剂可以实现选择性的C-O键裂解。这两种类型的过渡金属催化的交叉偶联反应代表了合成化学中的一个有吸引力的概念,因为酯和酰胺作为有机合成的前体普遍存在。此外,酰胺和酯的高稳定性在存在其他亲电试剂的情况下为正交交叉偶联策略提供了前所未有的机会。
更新日期:2018-09-21
中文翻译:

定义明确的钯(II)-NHC预催化剂,用于通过选择性NC-OC / C裂解进行酰胺和酯的交叉偶联反应
过渡金属催化的交叉偶联反应是合成化学家可快速构建C–C和C–X键的最有力工具。最近,在利用明确定义的Pd(II)-NHC络合物进行区域和化学选择性酰基C–X(X = N,O)裂解的酰胺和酯的交叉偶联反应的新兴领域,已取得了巨大进展。在钯催化的交叉偶联中使用N-杂环卡宾作为配体可以使酰胺和酯发生反应,而以前使用钯是不可能的,或者只有在苛刻的条件下才能实现。这些反应为合成化学家提供了一种诱人的方法,可以以钯催化固有的广泛交叉偶联通用性来操作传统上惰性的酰胺和酯键。稳定酰基亲电试剂的交叉偶联领域的研究可以按经历交叉偶联的亲电试剂的类型大致分类。最近的研究表明,过渡金属催化的酰胺交叉偶联是将经典惰性酰胺键转化为通用的酰基金属中间体的最直接,最广泛的方法之一,可以在交叉偶联反应中系统地利用该中间体。有机合成的新范式。实现该过程的高化学选择性的关键是控制酰胺共振(n 最近的研究表明,过渡金属催化的酰胺交叉偶联是将经典惰性酰胺键转化为通用的酰基金属中间体的最直接,最广泛的方法之一,可以在交叉偶联反应中系统地利用该中间体。有机合成的新范式。实现该过程高化学选择性的关键是控制酰胺共振(n 最近的研究表明,过渡金属催化的酰胺交叉偶联是将经典惰性酰胺键转化为通用的酰基金属中间体的最直接,最广泛的方法之一,可以在交叉偶联反应中系统地利用该中间体。有机合成的新范式。实现该过程的高化学选择性的关键是控制酰胺共振(nN到πC═O *共轭,旋转约ca。在平面酰胺中为15–20 kcal / mol),从而能够以合理且可预测的方式将N–C酰胺键氧化加成到金属上。这种催化方式已扩展到芳基酯的C(酰基)-O交叉偶联反应,其中通过合理匹配芳基酯亲电子试剂和亲核金属催化剂可以实现选择性的C-O键裂解。这两种类型的过渡金属催化的交叉偶联反应代表了合成化学中的一个有吸引力的概念,因为酯和酰胺作为有机合成的前体普遍存在。此外,酰胺和酯的高稳定性在存在其他亲电试剂的情况下为正交交叉偶联策略提供了前所未有的机会。

































 京公网安备 11010802027423号
京公网安备 11010802027423号