当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4‐[(1, 3‐Dioxoisoindolin‐2‐yl)methyl]benzenesulfonamide: Full Structural and Spectroscopic Characterization and Molecular Docking with Carbonic Anhydrase II
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-09-21 , DOI: 10.1002/slct.201802484 Halil Gökce 1 , Nuri Öztürk 2 , Yusuf Sert 3 , Adel S. El-Azab 4, 5 , Nawaf A. AlSaif 4 , Alaa A.-M. Abdel-Aziz 4, 6
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-09-21 , DOI: 10.1002/slct.201802484 Halil Gökce 1 , Nuri Öztürk 2 , Yusuf Sert 3 , Adel S. El-Azab 4, 5 , Nawaf A. AlSaif 4 , Alaa A.-M. Abdel-Aziz 4, 6
Affiliation
|
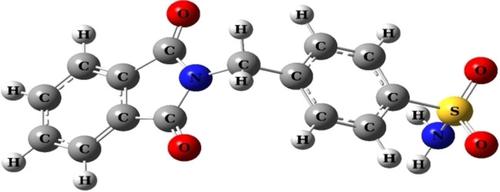
The structural, spectral, electronic, thermodynamic and nonlinear optical features of 4‐[(1, 3‐dioxoisoindolin‐2‐yl)methyl]benzenesulfonamide were investigated using experimental and theoretical methods. Experimental characterization was carried out via FT‐IR, Raman, carbon‐13 and proton NMR chemical shifts and UV‐Vis. spectroscopic techniques. The molecular electronic structure computations were done with DFT/B3LYP method using the 6–311++G(2d,2p) basis set. VEDA4 software was used to analyze in PED (potential energy distribution) to vibrational profile of computed harmonic frequencies. Hirshfeld surface analysis was performed to determine interactions in crystal packing of the compound. Additionally, the highest occupied molecular orbitals (HOMOs), lowest unoccupied molecular orbitals (LUMOs), nonlinear optical (NLO) analysis, molecular electrostatic potential (MEP and ESP) surfaces and thermochemical properties were studied with theoretical computational method. The localization contributions on the defined molecular groups of molecular orbitals in the electronic transitions were investigated. Enzyme‐ligand interactions of 4‐[(1, 3‐dioxoisoindolin‐2‐yl)methyl]benzenesulfonamide with carbonic anhydrase II were investigated by molecular docking analysis. The experimental and calculated structural and spectroscopic data were compared with each other. The correlation between computed results and experimental records is in a good harmony.
中文翻译:

4-[(1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺:完整的结构和光谱表征以及与碳酸酐酶的分子对接II
利用实验和理论方法研究了4-[((1,3-二氧代异吲哚啉-2--2-基)甲基]苯磺酰胺的结构,光谱,电子,热力学和非线性光学特征。通过FT-IR,拉曼,碳13和质子NMR化学位移和UV-Vis进行了实验表征。光谱技术。分子电子结构计算是使用DFT / B3LYP方法,使用6–311 ++ G(2d,2p)基集进行的。VEDA4软件用于分析PED(势能分布)中计算出的谐波频率的振动曲线。进行了Hirshfeld表面分析,以确定该化合物的晶体堆积中的相互作用。此外,最高占据分子轨道(HOMO),最低未占据分子轨道(LUMO),非线性光学(NLO)分析,用理论计算方法研究了分子静电势(MEP和ESP)表面和热化学性质。研究了在电子跃迁中分子轨道的确定分子组上的定位贡献。通过分子对接研究了4-[((1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺与碳酸酐酶II的酶-配体相互作用。实验和计算的结构和光谱数据相互比较。计算结果与实验记录之间的相关性很好。研究了在电子跃迁中分子轨道的确定分子组上的定位贡献。通过分子对接研究了4-[(1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺与碳酸酐酶II的酶-配体相互作用。实验和计算的结构和光谱数据相互比较。计算结果与实验记录之间的相关性很好。研究了在电子跃迁中分子轨道的确定分子组上的定位贡献。通过分子对接研究了4-[((1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺与碳酸酐酶II的酶-配体相互作用。实验和计算的结构和光谱数据相互比较。计算结果与实验记录之间的相关性很好。
更新日期:2018-09-21
中文翻译:

4-[(1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺:完整的结构和光谱表征以及与碳酸酐酶的分子对接II
利用实验和理论方法研究了4-[((1,3-二氧代异吲哚啉-2--2-基)甲基]苯磺酰胺的结构,光谱,电子,热力学和非线性光学特征。通过FT-IR,拉曼,碳13和质子NMR化学位移和UV-Vis进行了实验表征。光谱技术。分子电子结构计算是使用DFT / B3LYP方法,使用6–311 ++ G(2d,2p)基集进行的。VEDA4软件用于分析PED(势能分布)中计算出的谐波频率的振动曲线。进行了Hirshfeld表面分析,以确定该化合物的晶体堆积中的相互作用。此外,最高占据分子轨道(HOMO),最低未占据分子轨道(LUMO),非线性光学(NLO)分析,用理论计算方法研究了分子静电势(MEP和ESP)表面和热化学性质。研究了在电子跃迁中分子轨道的确定分子组上的定位贡献。通过分子对接研究了4-[((1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺与碳酸酐酶II的酶-配体相互作用。实验和计算的结构和光谱数据相互比较。计算结果与实验记录之间的相关性很好。研究了在电子跃迁中分子轨道的确定分子组上的定位贡献。通过分子对接研究了4-[(1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺与碳酸酐酶II的酶-配体相互作用。实验和计算的结构和光谱数据相互比较。计算结果与实验记录之间的相关性很好。研究了在电子跃迁中分子轨道的确定分子组上的定位贡献。通过分子对接研究了4-[((1,3-二氧代异吲哚啉-2-基)甲基]苯磺酰胺与碳酸酐酶II的酶-配体相互作用。实验和计算的结构和光谱数据相互比较。计算结果与实验记录之间的相关性很好。






























 京公网安备 11010802027423号
京公网安备 11010802027423号