当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced Intramolecular Charge Transfer and Hyperpolarizability Coefficient in Push-Pull Pyridinium Salts with Increasing Strength of the Acceptor Group.
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-10-18 , DOI: 10.1002/cplu.201800393
Alessio Cesaretti 1 , Carmela Bonaccorso 2 , Fausto Elisei 1 , Cosimo G Fortuna 2 , Letizia Mencaroni 1 , Anna Spalletti 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-10-18 , DOI: 10.1002/cplu.201800393
Alessio Cesaretti 1 , Carmela Bonaccorso 2 , Fausto Elisei 1 , Cosimo G Fortuna 2 , Letizia Mencaroni 1 , Anna Spalletti 1
Affiliation
|
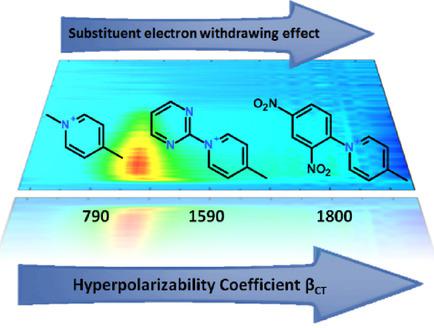
The synthesis of three push-pull cationic dyes is reported here together with a photophysical study carried out by stationary and ultrafast spectroscopies. The hyperpolarizability (β) values of the three molecules have been estimated through a simple solvatochromic method based on conventional, low-cost equipment, which had been tested previously with success in our laboratory. The investigated pyridinium salts showing strong negative solvatochromism bear the same piperidine ring as a strong electron-donor group and the same thiophenes as electron-rich π-linkers, but differ in terms of the N-substituent on the electron-acceptor pyridinium unit, namely N-methyl in compound A, N-pyrimidin-2yl in B and N-2,4-dinitrophenyl in C. The derived β values were found to increase (in the order A
中文翻译:

随着受体基团强度的增加,推挽式吡啶鎓盐中的光诱导分子内电荷转移和超极化系数。
此处报道了三种推挽阳离子染料的合成,以及通过固定和超快光谱学进行的光物理研究。这三种分子的超极化率(β)值是通过基于常规低成本设备的简单溶剂变色方法估算的,该方法先前已经在我们的实验室中成功进行了测试。所研究的吡啶鎓盐显示出很强的负溶剂变色性,与一个强电子给体基团具有相同的哌啶环,并且与富电子的π-连接基具有相同的噻吩,但在电子受体吡啶鎓单元上的N-取代基不同,即化合物A中的N-甲基,B中的N-嘧啶-2yl和C中的N-2,4-二硝基苯基。发现衍生的β值增加(顺序为A
更新日期:2018-10-18
中文翻译:

随着受体基团强度的增加,推挽式吡啶鎓盐中的光诱导分子内电荷转移和超极化系数。
此处报道了三种推挽阳离子染料的合成,以及通过固定和超快光谱学进行的光物理研究。这三种分子的超极化率(β)值是通过基于常规低成本设备的简单溶剂变色方法估算的,该方法先前已经在我们的实验室中成功进行了测试。所研究的吡啶鎓盐显示出很强的负溶剂变色性,与一个强电子给体基团具有相同的哌啶环,并且与富电子的π-连接基具有相同的噻吩,但在电子受体吡啶鎓单元上的N-取代基不同,即化合物A中的N-甲基,B中的N-嘧啶-2yl和C中的N-2,4-二硝基苯基。发现衍生的β值增加(顺序为A

































 京公网安备 11010802027423号
京公网安备 11010802027423号