当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Benzoxazine Atropisomers: Intrinsic Atropisomerization Mechanism and Conversion to High Performance Thermosets
Macromolecules ( IF 5.1 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acs.macromol.8b01924 Kan Zhang 1 , Zhikun Shang 1 , Corey J. Evans 2 , Lu Han 3 , Hatsuo Ishida 3 , Shengfu Yang 2
Macromolecules ( IF 5.1 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acs.macromol.8b01924 Kan Zhang 1 , Zhikun Shang 1 , Corey J. Evans 2 , Lu Han 3 , Hatsuo Ishida 3 , Shengfu Yang 2
Affiliation
|
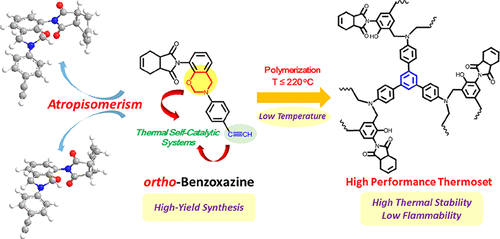
Atropisomers have inspired chemists and biologists for decades due to their chiral structures and associated biological properties. However, most of atropisomers reported so far arise in highly substituted biaryls and related compounds, and other types have been rarely observed. Here we report a new type of atropisomerism in ortho-tetrahydrophthalimide functional 1,3-benzoxazine family, where the atropisomerism is evident from NMR spectra, with the branching ratio of the atropisomeric configurations invariant with the measurement temperatures. Density functional theory calculations suggested that the reaction intermediate, ortho-tetrahydrophthalimide phenol, is key to the atropisomerism, which creates a large energy barrier after deprotonation and thus determines the branching ratios of the benzoxazine atropisomers. In addition, the ring-opening polymerization of benzoxazine atropisomers has also been investigated. The benzoxazine atropisomers bearing acetylene exhibit unexpectedly low polymerization temperature in the absence of catalysts, suggesting a self-catalyzed polymerization process. Despite the absence of antiflammable additives, the corresponding polybenzoxazine deriving from benzoxazine atropisomers containing acetylene shows exceptionally low heat release capacity (67.2 J g1–K–1) and excellent char residue value (62%). With this work we demonstrate atropisomerism in the 1,3-benzoxazine family for the first time, and provide molecular-level insights to the mechanism, which can open up possibilities for new applications of atropisomers spanning from the microelectronic to the aerospace industries.
中文翻译:

苯并恶嗪阻转异构体:固有的阻转异构化机理和向高性能热固性材料的转化
阻转异构体由于其手性结构和相关的生物学特性,已经启发了化学家和生物学家数十年。然而,迄今为止报道的大多数阻转异构体都出现在高度取代的联芳基和相关化合物中,很少观察到其他类型。在这里,我们报道了邻四氢邻苯二甲酰亚胺官能的1,3-苯并恶嗪家族中的一种新型的对映异构现象,其中从NMR光谱中可以看出其对映异构现象,其对映异构构型的分支比随测量温度而不变。密度泛函理论计算表明,反应中间体,邻四氢邻苯二甲酰亚胺苯酚是阻转异构现象的关键,它在去质子化后会形成较大的能垒,从而决定了苯并恶嗪阻转异构体的支化比。另外,还研究了苯并恶嗪阻转异构体的开环聚合。在不存在催化剂的情况下,带有乙炔的苯并恶嗪阻转异构体表现出出乎意料的低聚合温度,这表明其是自催化的聚合过程。尽管不存在抗燃添加剂,但衍生自含乙炔的苯并恶嗪阻转异构体的相应聚苯并恶嗪显示出极低的放热能力(67.2 J g 1– K –1)和极好的炭渣值(62%)。通过这项工作,我们首次证明了1,3-苯并恶嗪家族中的阻转异构现象,并为该机理提供了分子水平的见解,这为从微电子到航空航天工业的阻转异构体的新应用打开了可能性。
更新日期:2018-09-20
中文翻译:

苯并恶嗪阻转异构体:固有的阻转异构化机理和向高性能热固性材料的转化
阻转异构体由于其手性结构和相关的生物学特性,已经启发了化学家和生物学家数十年。然而,迄今为止报道的大多数阻转异构体都出现在高度取代的联芳基和相关化合物中,很少观察到其他类型。在这里,我们报道了邻四氢邻苯二甲酰亚胺官能的1,3-苯并恶嗪家族中的一种新型的对映异构现象,其中从NMR光谱中可以看出其对映异构现象,其对映异构构型的分支比随测量温度而不变。密度泛函理论计算表明,反应中间体,邻四氢邻苯二甲酰亚胺苯酚是阻转异构现象的关键,它在去质子化后会形成较大的能垒,从而决定了苯并恶嗪阻转异构体的支化比。另外,还研究了苯并恶嗪阻转异构体的开环聚合。在不存在催化剂的情况下,带有乙炔的苯并恶嗪阻转异构体表现出出乎意料的低聚合温度,这表明其是自催化的聚合过程。尽管不存在抗燃添加剂,但衍生自含乙炔的苯并恶嗪阻转异构体的相应聚苯并恶嗪显示出极低的放热能力(67.2 J g 1– K –1)和极好的炭渣值(62%)。通过这项工作,我们首次证明了1,3-苯并恶嗪家族中的阻转异构现象,并为该机理提供了分子水平的见解,这为从微电子到航空航天工业的阻转异构体的新应用打开了可能性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号