当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformation and Aggregation of Selectively PEGylated and Lipidated Gastric Peptide Hormone Human PYY3–36
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acs.biomac.8b01209 Valeria Castelletto 1 , Ian W. Hamley 1 , Jani Seitsonen 2 , Janne Ruokolainen 2 , Gemma Harris 3 , Kathrin Bellmann-Sickert 4 , Annette G. Beck-Sickinger 4
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acs.biomac.8b01209 Valeria Castelletto 1 , Ian W. Hamley 1 , Jani Seitsonen 2 , Janne Ruokolainen 2 , Gemma Harris 3 , Kathrin Bellmann-Sickert 4 , Annette G. Beck-Sickinger 4
Affiliation
|
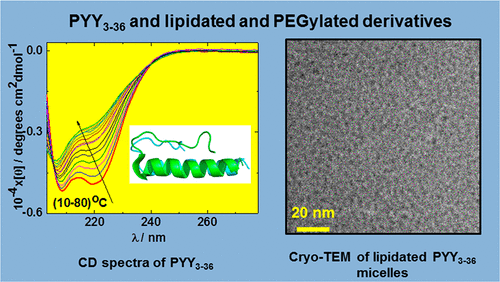
The gastric peptide hormone human PYY3–36 is a target for the development of therapeutics, especially for treatment of obesity. The conformation and aggregation behavior of PEGylated and lipidated derivatives of this peptide are examined using a combination of fluorescence dye assays, circular dichroism (CD) spectroscopy, analytical ultracentrifugation (AUC) measurements, small-angle X-ray scattering (SAXS) and cryogenic-transmission electron microscopy (cryo-TEM). The behavior of two PYY3–36 derivatives lipidated (with octyl chains) in different positions is compared to that of two derivatives with PEG attached at different residues and to that of the native peptide. We find that, unexpectedly, PYY3–36 forms amyloid fibril structures above a critical aggregation concentration. Formation of these structures is suppressed by PEGylation or lipidation. PEGylation significantly reduces the (reversible) loss of α-helix content observed on heating PYY3–36. The PEG conjugates form mainly monomeric structures in solution- coiled-coil formation, and other aggregation presumably being sterically hindered by swollen PEG chains. However, some small aggregates are detected by AUC. In complete contrast, both of the two lipidated peptides show the formation of spherical micelle-like structures which are small oligomeric aggregates. Our findings show that PEGylation and lipidation are complementary strategies to tune the conformation and aggregation of the important gastric peptide hormone human PYY3–36.
中文翻译:

选择性聚乙二醇化和脂化胃肽激素人类PYY 3–36的构象和聚集
胃肽激素人类PYY 3–36是治疗药物开发的目标,尤其是治疗肥胖症。使用荧光染料测定,圆二色性(CD)光谱,分析超速离心(AUC)测量,小角度X射线散射(SAXS)和超低温-透射电子显微镜(cryo-TEM)。将两种在不同位置脂化(带有辛基链)的PYY 3–36衍生物的行为与两种在不同残基处连接了PEG的衍生物和天然肽的行为进行了比较。我们意外地发现PYY 3–36形成高于临界聚集浓度的淀粉样蛋白原纤维结构。这些结构的形成被聚乙二醇化或脂化抑制。聚乙二醇化可显着降低加热PYY 3–36时观察到的(可逆)α-螺旋含量的损失。PEG缀合物在溶液卷曲螺旋形成中主要形成单体结构,而其他聚集可能在空间上受PEG链溶胀的阻碍。但是,AUC检测到一些小的聚集体。完全相反,两种脂化的肽都显示出球形胶束样结构,是小的寡聚体。我们的发现表明,聚乙二醇化和脂化是补充策略,可调节重要的胃肽激素人类PYY 3–36的构象和聚集。
更新日期:2018-09-19
中文翻译:

选择性聚乙二醇化和脂化胃肽激素人类PYY 3–36的构象和聚集
胃肽激素人类PYY 3–36是治疗药物开发的目标,尤其是治疗肥胖症。使用荧光染料测定,圆二色性(CD)光谱,分析超速离心(AUC)测量,小角度X射线散射(SAXS)和超低温-透射电子显微镜(cryo-TEM)。将两种在不同位置脂化(带有辛基链)的PYY 3–36衍生物的行为与两种在不同残基处连接了PEG的衍生物和天然肽的行为进行了比较。我们意外地发现PYY 3–36形成高于临界聚集浓度的淀粉样蛋白原纤维结构。这些结构的形成被聚乙二醇化或脂化抑制。聚乙二醇化可显着降低加热PYY 3–36时观察到的(可逆)α-螺旋含量的损失。PEG缀合物在溶液卷曲螺旋形成中主要形成单体结构,而其他聚集可能在空间上受PEG链溶胀的阻碍。但是,AUC检测到一些小的聚集体。完全相反,两种脂化的肽都显示出球形胶束样结构,是小的寡聚体。我们的发现表明,聚乙二醇化和脂化是补充策略,可调节重要的胃肽激素人类PYY 3–36的构象和聚集。































 京公网安备 11010802027423号
京公网安备 11010802027423号