当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Modeling of 2-Amino-4,6-dichoropyrimidine in Ten Pure Solvents and (Ethyl Acetate + Ethanol) Solvent Mixtures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-09-19 , DOI: 10.1021/acs.jced.8b00292 Shasha Li 1, 2 , Yong Liu 1, 2 , Fan Yin 1, 2 , Xiangyue Ye 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-09-19 , DOI: 10.1021/acs.jced.8b00292 Shasha Li 1, 2 , Yong Liu 1, 2 , Fan Yin 1, 2 , Xiangyue Ye 1, 2
Affiliation
|
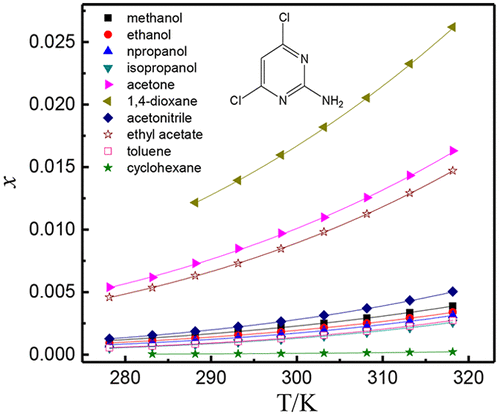
The basis of purification and further theoretical studies of 2-amino-4,6-dichoropyrimidine is the solubility and solution thermodynamics in different solvents. In this paper, the solubility of 2-amino-4,6-dichoropyrimidine in 10 neat solvents (methanol, ethanol, n-propanol, isopropanol, acetone, acetonitrile, ethyl acetate, 1,4-dioxane, toluene, and cyclohexane) and binary liquid mixtures (ethyl acetate + ethanol) was measured by the isothermal saturation method at temperatures range from 278.15 to 318.15 K, at p = 101.2 kPa. High-performance liquid chromatography (HPLC) was used to analyze the solubility of 2-amino-4,6-dichoropyrimidine in selected solvents. To sum up, the equilibrium mole fraction solubility was highest in 1,4-dioxane (2.619 × 10–2 at 318.15 K) and lowest in cyclohexane (0.02238 × 10–2 at 318.15 K), and ranked as 1,4-dioxane (2.619 × 10–2 at 318.15 K) > acetone (1.630 × 10–2 at 318.15 K) > ethyl acetate (1.471 × 10–2 at 318.15 K) > acetonitrile (0.5048 × 10–2 at 318.15 K) > methanol (0.3888 × 10–2 at 318.15 K) > ethanol (0.3391 × 10–2 at 318.15 K) > n-propanol (0.3133 × 10–2 at 318.15 K) > toluene (0.2737 × 10–2 at 318.15 K) > isopropanol (0.2574 × 10–2 at 318.15 K) > cyclohexane (0.02238 × 10–2 at 318.15 K). The achieved solubility values in monosolvents were correlated by the modified Apelblat equation, λh equation, Wilson model, and NRTL model and in solvent mixtures by three cosolvency models including Jouyban-Acree model, van’t Hoff-Jouyban-Acree model, and Apelblat-Jouyban-Acree model. Calculated results fitted well with experimental data. Consequently, for the monosolvents, the values of root-mean-square deviation (RMSD) and relative average deviation (RAD) were less than 0.58 × 10–4 and 0.80%, respectively, and for the binary solvent mixtures were 0.49 × 10–4 and 0.46%. Furthermore, the Gibbs energy, mixing enthalpy, mixing entropy, activity coefficient at infinitesimal concentration (γ1∞) and reduced excess enthalpy (H1E,∞) in monosolvents, and dissolution property in mixed solvents were computed. The mixing process of 2-amino-4,6-dichoropyrimidine in the studied solvents was spontaneous and endothermic. The obtained solubility and thermodynamic studies would be used to optimize the purification process of 2-amino-4,6-dichoropyrimidine.
中文翻译:

2-氨基-4,6-二胆嘧啶在十种纯溶剂和(乙酸乙酯+乙醇)溶剂混合物中的溶解度测量和建模
2-氨基-4,6-二胆嘧啶的纯化和进一步理论研究的基础是在不同溶剂中的溶解度和溶液热力学。在本文中,2-氨基-4,6-二胆嘧啶在10种纯溶剂(甲醇,乙醇,正丙醇,异丙醇,丙酮,乙腈,乙酸乙酯,1,4-二恶烷,甲苯和环己烷)中的溶解度和通过等温饱和法在278.15至318.15 K的温度范围(p = 101.2 kPa )下测量二元液体混合物(乙酸乙酯+乙醇)。高效液相色谱(HPLC)用于分析2-氨基-4,6-二胆嘧啶在所选溶剂中的溶解度。综上所述,平衡摩尔分数溶解度在1,4-二恶烷中最高(2.619×10 –2在318.15 K时为最低),在环己烷中最低(在318.15 K时为0.02238×10 -2),排名为1,4-二恶烷(在318.15 K时为2.619×10 –2)>丙酮(在318.15 K时为1.630×10 -2)>乙酸乙酯(318.15 K时为1.471×10 –2)>乙腈(318.15 K为0.5048×10 –2)>甲醇(318.15 K为0.3888×10 –2)>乙醇(318.15 K为0.3391×10 –2)> n丙醇(在318.15 K时为0.3133×10 –2)>甲苯(在318.15 K时为0.2737×10 –2)>异丙醇(在318.15 K时为0.2574×10 –2)>环己烷(0.02238×10 –2在318.15 K)。通过修改后的Apelblat方程,λh方程,Wilson模型和NRTL模型,以及通过三种共溶模型(包括Jouyban-Acree模型,van't Hoff-Jouyban-Acree模型和Apelblat- Jouyban-Acree模型。计算结果与实验数据非常吻合。因此,对于单溶剂,均方根偏差(RMSD)和相对平均偏差(RAD)的值分别小于0.58×10 –4和0.80%,对于二元溶剂混合物为0.49×10 – 4和0.46%。此外,吉布斯自由能,焓混合,在无穷小的浓度(混合熵,活度系数γ 1 ∞)并降低了单溶剂中的过量焓(H 1 E,∞),并计算了在混合溶剂中的溶解性。2-氨基-4,6-二胆嘧啶在所研究的溶剂中的混合过程是自发的并且是吸热的。获得的溶解度和热力学研究将用于优化2-氨基-4,6-二胆嘧啶的纯化工艺。
更新日期:2018-09-20
中文翻译:

2-氨基-4,6-二胆嘧啶在十种纯溶剂和(乙酸乙酯+乙醇)溶剂混合物中的溶解度测量和建模
2-氨基-4,6-二胆嘧啶的纯化和进一步理论研究的基础是在不同溶剂中的溶解度和溶液热力学。在本文中,2-氨基-4,6-二胆嘧啶在10种纯溶剂(甲醇,乙醇,正丙醇,异丙醇,丙酮,乙腈,乙酸乙酯,1,4-二恶烷,甲苯和环己烷)中的溶解度和通过等温饱和法在278.15至318.15 K的温度范围(p = 101.2 kPa )下测量二元液体混合物(乙酸乙酯+乙醇)。高效液相色谱(HPLC)用于分析2-氨基-4,6-二胆嘧啶在所选溶剂中的溶解度。综上所述,平衡摩尔分数溶解度在1,4-二恶烷中最高(2.619×10 –2在318.15 K时为最低),在环己烷中最低(在318.15 K时为0.02238×10 -2),排名为1,4-二恶烷(在318.15 K时为2.619×10 –2)>丙酮(在318.15 K时为1.630×10 -2)>乙酸乙酯(318.15 K时为1.471×10 –2)>乙腈(318.15 K为0.5048×10 –2)>甲醇(318.15 K为0.3888×10 –2)>乙醇(318.15 K为0.3391×10 –2)> n丙醇(在318.15 K时为0.3133×10 –2)>甲苯(在318.15 K时为0.2737×10 –2)>异丙醇(在318.15 K时为0.2574×10 –2)>环己烷(0.02238×10 –2在318.15 K)。通过修改后的Apelblat方程,λh方程,Wilson模型和NRTL模型,以及通过三种共溶模型(包括Jouyban-Acree模型,van't Hoff-Jouyban-Acree模型和Apelblat- Jouyban-Acree模型。计算结果与实验数据非常吻合。因此,对于单溶剂,均方根偏差(RMSD)和相对平均偏差(RAD)的值分别小于0.58×10 –4和0.80%,对于二元溶剂混合物为0.49×10 – 4和0.46%。此外,吉布斯自由能,焓混合,在无穷小的浓度(混合熵,活度系数γ 1 ∞)并降低了单溶剂中的过量焓(H 1 E,∞),并计算了在混合溶剂中的溶解性。2-氨基-4,6-二胆嘧啶在所研究的溶剂中的混合过程是自发的并且是吸热的。获得的溶解度和热力学研究将用于优化2-氨基-4,6-二胆嘧啶的纯化工艺。































 京公网安备 11010802027423号
京公网安备 11010802027423号