Separation and Purification Technology ( IF 8.1 ) Pub Date : 2018-09-18 , DOI: 10.1016/j.seppur.2018.09.051
Liuyang Dong , Fen Jiao , Wenqing Qin , Hailing Zhu , Wenhao Jia
|
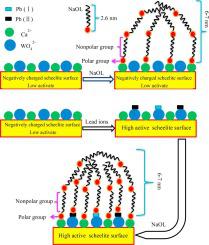
This paper systematically studied the effect of lead ions on floatability of scheelite under sodium oleate (NaOL) system, and the deep adsorption mechanism was revealed by a series of mechanism experiments and the adsorption morphology of NaOL was observed by atomic force microscope (AFM). Micro-flotation test indicated that the floatability of scheelite increased sharply from 47.25% to 80.12% in the presence of lead ions at pH 7. Zeta potential results revealed the positively charged lead ions species (Pb2+ and PbOH+) positively changed the zeta potential of scheelite, which favored the adsorption of negatively charged NaOL species (RCOO−, RCOOH·RCOO− etc.). XPS and FTIR analysis confirmed that NaOL was chemisorbed on scheelite surface and the adsorption was more severe in the presence of lead ions. Lead ions were chemisorbed on scheelite surface in the form of W-O-Pb2+, which facilitated the further adsorption of NaOL. This increased the active sites on scheelite surface for NaOL adsorption. AFM images intuitively showed the presence of lead ions increased the adsorption coverage of NaOL but did not affect the thickness of the adsorption layer. The adsorption thickness was still 6 nm–7 nm and the adsorption was multilayer adsorption. Based on these studies, an adsorption model was finally obtained.
中文翻译:

铅离子对白钨矿浮选的活化作用:吸附机理、AFM成像和吸附模型
本文系统研究了油酸钠(NaOL)体系下铅离子对白钨矿可浮性的影响,通过一系列机理实验揭示了其深层吸附机理,并通过原子力显微镜(AFM)观察了NaOL的吸附形貌。微浮选测试表明,在 pH 7 的铅离子存在下,白钨矿的可浮性从 47.25% 急剧增加至 80.12%。Zeta 电位结果显示,带正电荷的铅离子物种(Pb 2+和 PbOH +)对 zeta 产生正向变化白钨矿的电位,有利于带负电的NaOL物质(RCOO -,RCOOH·RCOO -等)的吸附。 XPS和FTIR分析证实NaOL在白钨矿表面发生化学吸附,且在铅离子存在下吸附更加严重。铅离子以WO-Pb 2+的形式化学吸附在白钨矿表面,有利于NaOL的进一步吸附。这增加了白钨矿表面用于 NaOL 吸附的活性位点。 AFM图像直观地表明铅离子的存在增加了NaOL的吸附覆盖率,但不影响吸附层的厚度。吸附厚度仍为6 nm~7 nm,吸附为多层吸附。基于这些研究,最终获得了吸附模型。































 京公网安备 11010802027423号
京公网安备 11010802027423号