当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Near-Barrierless Ammonium Bisulfate Formation via a Loop-Structure Promoted Proton-Transfer Mechanism on the Surface of Water
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-02-04 , DOI: 10.1021/jacs.5b13048 Lei Li 1 , Manoj Kumar 1 , Chongqin Zhu 1 , Jie Zhong 1 , Joseph S. Francisco 1 , Xiao Cheng Zeng 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-02-04 , DOI: 10.1021/jacs.5b13048 Lei Li 1 , Manoj Kumar 1 , Chongqin Zhu 1 , Jie Zhong 1 , Joseph S. Francisco 1 , Xiao Cheng Zeng 1, 2
Affiliation
|
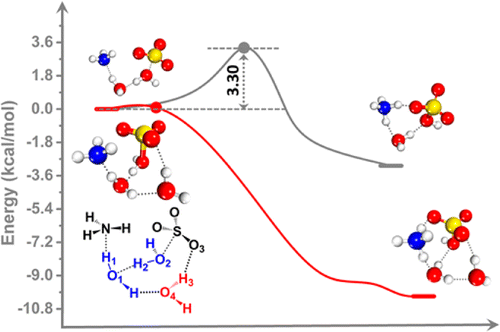
In the atmosphere, a well-known and conventional pathway toward the formation of ammonium sulfate is through the neutralization of sulfuric acid with ammonia (NH3) in water droplets. Here, we present direct ab initio molecular dynamics simulation evidence of the formation of ammonium bisulfate (NH4HSO4) from the hydrated NH3 and SO3 molecules in a water trimer as well as on the surface of a water droplet. This reaction suggests a new mechanism for the formation of ammonium sulfate in the atmosphere, especially when the concentration of NH3 is high (e.g., ∼10 μg m(-3)) in the air. Contrary to the water monomer and dimer, the water trimer enables near-barrierless proton transfer via the formation of a unique loop structure around the reaction center. The formation of the loop structure promotes the splitting of a water molecule in the proton-transfer center, resulting in the generation a NH4(+)/HSO4(-) ion pair. The loop-structure promoted proton-transfer mechanism is expected to be ubiquitous on the surface of cloud droplets with adsorbed NH3 and SO3 molecules and, thus, may play an important role in the nucleation of aerosol particles (e.g., fine particles PM2.5) in water droplets.
中文翻译:

通过环结构促进水表面质子转移机制形成近乎无障碍的硫酸氢铵
在大气中,形成硫酸铵的一种众所周知的常规途径是用水滴中的氨 (NH3) 中和硫酸。在这里,我们提出了从水三聚体中以及水滴表面上的水合 NH3 和 SO3 分子形成硫酸氢铵 (NH4HSO4) 的直接从头分子动力学模拟证据。该反应提出了一种在大气中形成硫酸铵的新机制,尤其是当空气中的 NH3 浓度很高(例如,约 10 μg m(-3))时。与水单体和二聚体相反,水三聚体通过在反应中心周围形成独特的环结构来实现近乎无障碍的质子转移。环结构的形成促进了质子转移中心中水分子的分裂,从而产生了 NH4(+)/HSO4(-) 离子对。环结构促进质子转移机制预计普遍存在于吸附有 NH3 和 SO3 分子的云滴表面,因此可能在气溶胶颗粒(例如细颗粒 PM2.5)的成核中起重要作用在水滴中。
更新日期:2016-02-04
中文翻译:

通过环结构促进水表面质子转移机制形成近乎无障碍的硫酸氢铵
在大气中,形成硫酸铵的一种众所周知的常规途径是用水滴中的氨 (NH3) 中和硫酸。在这里,我们提出了从水三聚体中以及水滴表面上的水合 NH3 和 SO3 分子形成硫酸氢铵 (NH4HSO4) 的直接从头分子动力学模拟证据。该反应提出了一种在大气中形成硫酸铵的新机制,尤其是当空气中的 NH3 浓度很高(例如,约 10 μg m(-3))时。与水单体和二聚体相反,水三聚体通过在反应中心周围形成独特的环结构来实现近乎无障碍的质子转移。环结构的形成促进了质子转移中心中水分子的分裂,从而产生了 NH4(+)/HSO4(-) 离子对。环结构促进质子转移机制预计普遍存在于吸附有 NH3 和 SO3 分子的云滴表面,因此可能在气溶胶颗粒(例如细颗粒 PM2.5)的成核中起重要作用在水滴中。

































 京公网安备 11010802027423号
京公网安备 11010802027423号