当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amyloid β-Protein Assembly and Alzheimer’s Disease: Dodecamers of Aβ42, but Not of Aβ40, Seed Fibril Formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-02-04 , DOI: 10.1021/jacs.5b11913 Nicholas J. Economou 1 , Maxwell J. Giammona 1 , Thanh D. Do 1 , Xueyun Zheng 1 , David B. Teplow 2 , Steven K. Buratto 1 , Michael T. Bowers 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-02-04 , DOI: 10.1021/jacs.5b11913 Nicholas J. Economou 1 , Maxwell J. Giammona 1 , Thanh D. Do 1 , Xueyun Zheng 1 , David B. Teplow 2 , Steven K. Buratto 1 , Michael T. Bowers 1
Affiliation
|
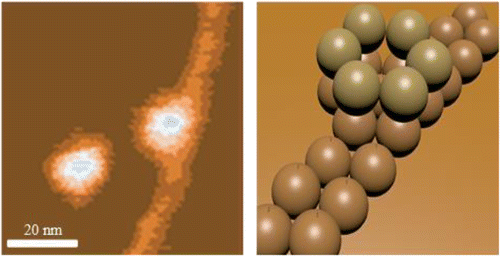
Evidence suggests that oligomers of the 42-residue form of the amyloid β-protein (Aβ), Aβ42, play a critical role in the etiology of Alzheimer's disease (AD). Here we use high resolution atomic force microscopy to directly image populations of small oligomers of Aβ42 that occur at the earliest stages of aggregation. We observe features that can be attributed to a monomer and to relatively small oligomers, including dimers, hexamers, and dodecamers. We discovered that Aβ42 hexamers and dodecamers quickly become the dominant oligomers after peptide solubilization, even at low (1 μM) concentrations and short (5 min) incubation times. Soon after (≥10 min), dodecamers are observed to seed the formation of extended, linear preprotofibrillar β-sheet structures. The preprotofibrils are a single Aβ42 layer in height and can extend several hundred nanometers in length. To our knowledge this is the first report of structures of this type. In each instance the preprotofibril is associated off center with a single layer of a dodecamer. Protofibril formation continues at longer times, but is accompanied by the formation of large, globular aggregates. Aβ40, by contrast, does not significantly form the hexamer or dodecamer but instead produces a mixture of smaller oligomers. These species lead to the formation of a branched chain-like network rather than discrete structures.
中文翻译:

β-淀粉样蛋白组装和阿尔茨海默病:Aβ42 的十二聚体,而不是 Aβ40 的十二聚体,种子原纤维的形成
有证据表明,淀粉样蛋白 β 蛋白 (Aβ) 的 42 个残基形式的寡聚体 Aβ42 在阿尔茨海默病 (AD) 的病因学中起关键作用。在这里,我们使用高分辨率原子力显微镜对发生在聚集最早阶段的 Aβ42 小寡聚体群体进行直接成像。我们观察到可归因于单体和相对较小的低聚物(包括二聚体、六聚体和十二聚体)的特征。我们发现 Aβ42 六聚体和十二聚体在肽溶解后迅速成为主要寡聚体,即使在低 (1 μM) 浓度和短 (5 分钟) 孵育时间下也是如此。不久之后(≥10 分钟),观察到十二聚体形成扩展的线性前原纤维 β-折叠结构。前原纤维在高度上是单个 Aβ42 层,长度可以延伸数百纳米。据我们所知,这是此类结构的第一份报告。在每种情况下,前原纤维都偏离中心与单层十二聚体相关联。原纤维的形成持续更长的时间,但伴随着大的球状聚集体的形成。相比之下,Aβ40 不会显着形成六聚体或十二聚体,而是产生较小的低聚物的混合物。这些物种导致形成支链状网络而不是离散结构。但伴随着大型球状聚集体的形成。相比之下,Aβ40 不会显着形成六聚体或十二聚体,而是产生较小的低聚物的混合物。这些物种导致形成支链状网络而不是离散结构。但伴随着大型球状聚集体的形成。相比之下,Aβ40 不会显着形成六聚体或十二聚体,而是产生较小的低聚物的混合物。这些物种导致形成支链状网络而不是离散结构。
更新日期:2016-02-04
中文翻译:

β-淀粉样蛋白组装和阿尔茨海默病:Aβ42 的十二聚体,而不是 Aβ40 的十二聚体,种子原纤维的形成
有证据表明,淀粉样蛋白 β 蛋白 (Aβ) 的 42 个残基形式的寡聚体 Aβ42 在阿尔茨海默病 (AD) 的病因学中起关键作用。在这里,我们使用高分辨率原子力显微镜对发生在聚集最早阶段的 Aβ42 小寡聚体群体进行直接成像。我们观察到可归因于单体和相对较小的低聚物(包括二聚体、六聚体和十二聚体)的特征。我们发现 Aβ42 六聚体和十二聚体在肽溶解后迅速成为主要寡聚体,即使在低 (1 μM) 浓度和短 (5 分钟) 孵育时间下也是如此。不久之后(≥10 分钟),观察到十二聚体形成扩展的线性前原纤维 β-折叠结构。前原纤维在高度上是单个 Aβ42 层,长度可以延伸数百纳米。据我们所知,这是此类结构的第一份报告。在每种情况下,前原纤维都偏离中心与单层十二聚体相关联。原纤维的形成持续更长的时间,但伴随着大的球状聚集体的形成。相比之下,Aβ40 不会显着形成六聚体或十二聚体,而是产生较小的低聚物的混合物。这些物种导致形成支链状网络而不是离散结构。但伴随着大型球状聚集体的形成。相比之下,Aβ40 不会显着形成六聚体或十二聚体,而是产生较小的低聚物的混合物。这些物种导致形成支链状网络而不是离散结构。但伴随着大型球状聚集体的形成。相比之下,Aβ40 不会显着形成六聚体或十二聚体,而是产生较小的低聚物的混合物。这些物种导致形成支链状网络而不是离散结构。













































 京公网安备 11010802027423号
京公网安备 11010802027423号