当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CeOx-Decorated NiFe-Layered Double Hydroxide for Efficient Alkaline Hydrogen Evolution by Oxygen Vacancy Engineering
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acsami.8b11688 Xixi Wang 1 , Yu Yang 2 , Lechen Diao 1 , Yu Tang 1 , Fang He 1 , Enzuo Liu 1 , Chunnian He 1 , Chunsheng Shi 1 , Jiajun Li 1 , Junwei Sha 1 , Shuaihua Ji 3 , Ping Zhang 2 , Liying Ma 1 , Naiqin Zhao 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acsami.8b11688 Xixi Wang 1 , Yu Yang 2 , Lechen Diao 1 , Yu Tang 1 , Fang He 1 , Enzuo Liu 1 , Chunnian He 1 , Chunsheng Shi 1 , Jiajun Li 1 , Junwei Sha 1 , Shuaihua Ji 3 , Ping Zhang 2 , Liying Ma 1 , Naiqin Zhao 1
Affiliation
|
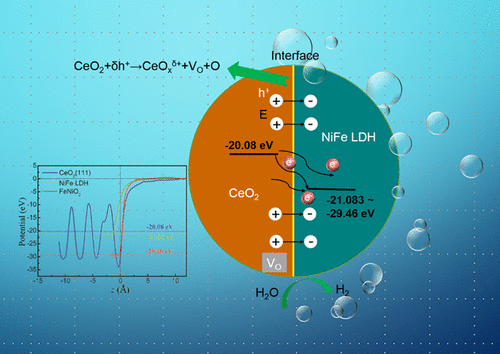
As a promising bifunctional electrocatalyst for water splitting, NiFe-layered double hydroxide (NiFe LDH) demonstrates an excellent activity toward oxygen evolution reaction (OER) in alkaline solution. However, its hydrogen evolution reaction (HER) activity is challenged owing to the poor electronic conductivity and insufficient electrochemical active sites. Therefore, a three-dimensional self-supporting metal hydroxide/oxide electrode with abundant oxygen vacancies is prepared by electrodepositing CeOx nanoparticles on NiFe LDH nanosheets. According to the density functional theory calculations and experimental studies, the oxygen vacancies at the NiFe LDH/CeOx interface can be introduced successfully because of the positive charges accumulation resulting from the local electron potential difference between NiFe LDH and CeOx. The oxygen vacancies accelerate the electron/ion migration rates, facilitate the charge transfer, and increase the electrochemical active sites, which give rise to an efficient activity toward HER in alkaline solution. Furthermore, [email protected] LDH/CeOx needs a lower potential of 1.51 V to drive a current density of 10 mA cm–2 in overall water splitting and demonstrates a superior performance compared with the benchmark Pt/C and RuO2, which is indicated to be a promising bifunctional electrode catalyst.
中文翻译:

CeO x修饰的NiFe层状双氢氧化物通过氧空位工程有效地释放碱性氢
作为一种有前景的用于水分解的双功能电催化剂,NiFe层状双氢氧化物(NiFe LDH)在碱性溶液中表现出优异的抗氧析出反应(OER)的活性。然而,由于不良的电子电导率和不足的电化学活性位,其氢释放反应(HER)活性受到挑战。因此,通过将CeO x纳米粒子电沉积在NiFe LDH纳米片上,制备了具有大量氧空位的三维自支撑金属氢氧化物/氧化物电极。根据密度泛函理论计算和实验研究,NiFe LDH / CeO x处的氧空位NiFe LDH和CeO x之间的局部电子势差引起的正电荷积累,可以成功引入介面。氧空位加速了电子/离子迁移速率,促进了电荷转移,并增加了电化学活性位,从而在碱性溶液中产生了对HER的有效活性。此外,[电子邮件保护的] LDH / CeO x在整个水分解过程中需要较低的电位1.51 V来驱动10 mA cm –2的电流密度,并且与基准的Pt / C和RuO 2相比,具有优越的性能,表明是有希望的双功能电极催化剂。
更新日期:2018-09-18
中文翻译:

CeO x修饰的NiFe层状双氢氧化物通过氧空位工程有效地释放碱性氢
作为一种有前景的用于水分解的双功能电催化剂,NiFe层状双氢氧化物(NiFe LDH)在碱性溶液中表现出优异的抗氧析出反应(OER)的活性。然而,由于不良的电子电导率和不足的电化学活性位,其氢释放反应(HER)活性受到挑战。因此,通过将CeO x纳米粒子电沉积在NiFe LDH纳米片上,制备了具有大量氧空位的三维自支撑金属氢氧化物/氧化物电极。根据密度泛函理论计算和实验研究,NiFe LDH / CeO x处的氧空位NiFe LDH和CeO x之间的局部电子势差引起的正电荷积累,可以成功引入介面。氧空位加速了电子/离子迁移速率,促进了电荷转移,并增加了电化学活性位,从而在碱性溶液中产生了对HER的有效活性。此外,[电子邮件保护的] LDH / CeO x在整个水分解过程中需要较低的电位1.51 V来驱动10 mA cm –2的电流密度,并且与基准的Pt / C和RuO 2相比,具有优越的性能,表明是有希望的双功能电极催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号